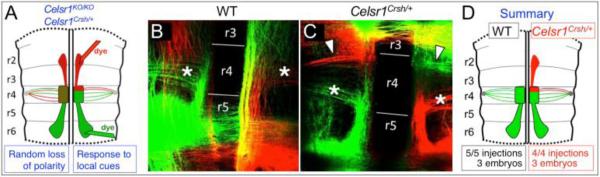
Figure 6. The rostrally migrating FBM neurons in Celsr1Crsh/+ embryos originate in the rostral portion of r4, adjacent to the r3/r4 boundary.
A, Schematic of a flat-mounted E12.5 hindbrain indicating the dye application sites within the rostral (NeuroVue Maroon, red) and caudal (NeuroVue Jade, green) migrating streams of FBM neurons to anterogradely label the FBM axons exiting in r4. Two hypotheses (blue boxes) and predicted phenotypes are shown. If the rostral migration phenotype of Celsr1 mutants results from a loss of polarity in FBM neurons in r4 (left side), a newborn FBM neuron in any location within r4 (mixed red/green stipples) could potentially migrate rostrally, resulting in a mixed collection of FBM axons exiting from r4. However if rostrally migrating neurons arise exclusively from the anterior margin of r4 in response to guidance cues unmasked in the mutants (right side), the FBM axons extended by the rostrally and caudally migrating neurons would be clearly segregated in r4. B-C, Flat-mounted views of dye-labeled WT and Celsr1Crsh/+ hindbrains. To maximize data collection from each embryo, the migratory streams were labeled on both sides (caudal left-green; rostral left-red; caudal right-red; rostral right-green). In a WT embryo (B), dye application in r5/r6 anterogradely labels FBM axons (asterisks) spanning the anterior-posterior extent of r4. Dye application in r2/r3 does not label any axon projections into the r4 exit points. In a Celsr1Crsh/+ embryo (C), dye application in r5/r6 anterogradely labels FBM axons in r4 (asterisks). Importantly, dye application in r2/r3 anterogradely labels FBM axons projecting toward the r4 exit points, extending along the anterior margin of r4 (arrowheads). Axons crossing the midline in r5 (B) and r3 (C) are commissural axons that were inadvertently labeled in these particular embryos, and are not relevant to the rostral migration defect. D, Schematic summarizing the results shown in B and C.