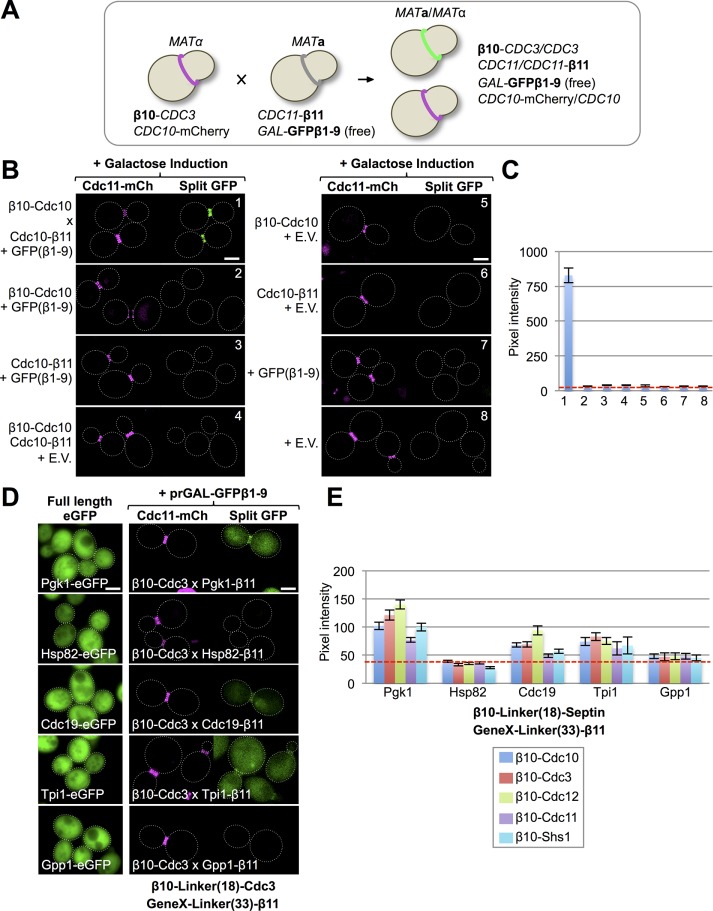
FIGURE 1:
Implementation of the tripartite split-GFP system in live yeast. (A) A mating-based strategy to generate cells expressing all of the components of the tripartite split-GFP method. A haploid that expresses an N-terminally β10-tagged protein of interest from its endogenous promoter at its native chromosomal locus (and also expresses an independent marker for the subcellular location of interest, where available) is mated to a haploid of opposite mating type that expresses a C-terminally β11-tagged protein of interest from its endogenous promoter at its native chromosomal locus and also harbors a CEN plasmid that expresses from a regulatable promoter the GFPβ1-9 barrel. (B) All three components of the tripartite split-GFP system are required to generate a protein–protein interaction signal in vivo. Diploids strains (1–8; Supplemental Table S3) that express all three or only two, one, or no components of the tripartite split-GFP system, as indicated, were constructed as in A by two rounds of selection on minimal (SD)-Leu-His medium with 2% glucose. GFPβ1-9 was carried on a LEU2-marked CEN plasmid (pGF-IVL794) and expressed under control of the GAL1/10 promoter. Cultures of the indicated diploids were grown overnight to saturation in SD-Leu-His medium with 2% raffinose–0.2% sucrose, back-diluted into the same medium with 2% galactose, grown at 30°C for 4.5 h, harvested, washed, and imaged by fluorescence microscopy. All images were captured after the identical exposure time and processed using ImageJ. Dotted white line, cell periphery. Scale bar, 2 μm. E.V., empty vector (pRS315). Diploid 1 contained one copy of β10-(32-residue linker)-Cdc10 and one copy of Cdc10-(33-residue linker)-β11 (Supplemental Figure S1). (C) Quantification of the average GFP fluorescence at the bud neck in budded cells (25–100 per culture) for the strains in B. Error bar, SEM. Dashed red line, average intrinsic background fluorescence (∼35–40 pixels) at the bud neck in cells lacking the components of the tripartite split-GFP system for images taken at the identical exposure time. (D) Left, confirmation that the five proteins indicated are abundant cytosolic proteins. Strains expressing each of the indicated proteins (see Supplemental Table S4) as an eGFP fusion (GFY-1977, GFY-1981, GFY-2034, GFY-2030, and GFY-2033) were grown to saturation in rich (YPD) medium, back-diluted into fresh YPD, grown to mid exponential phase, and imaged as in A, except that the exposure times varied: Gpp1-eGFP (150 ms), Hsp82-eGFP (150 ms), Cdc19-eGFP (100 ms), Pgk1-eGFP (50 ms), and Tpi1-eGFP (50 ms). Right, none of five extremely abundant cytosolic proteins exhibits more than a very weak interaction with any septin. Representative examples for the interaction of the indicated β11-tagged proteins with β10-Cdc3 using the tripartite split-GFP method. In diploids 164, 170, 272, 282, and 277 expressing the indicated proteins, expression of GFPβ1-9 was induced and the cells imaged as in B. (E) Quantification, as in C, of the fluorescence signal at the bud neck in dividing diploids (25–100 per culture) that expressed each abundant β11-tagged cytosolic protein with each β10-septin (see Supplemental Table S3 for a complete list).