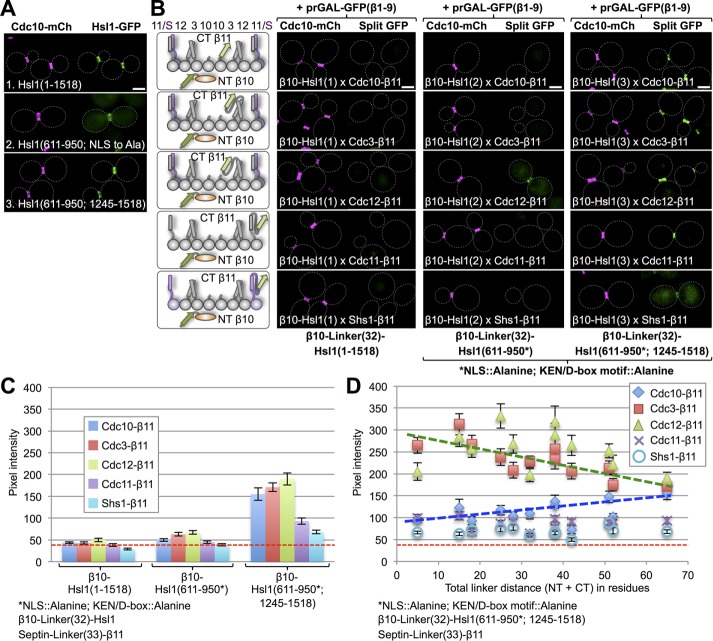
FIGURE 5:
Septin-binding domain of checkpoint kinase Hsl1 associates preferentially with Cdc3 and Cdc12. (A) Cells (strain GFY-42) expressing Cdc10-mCh and coexpressing from CEN plasmids, as indicated, 1) full-length Hsl1-eGFP (pGF-IVL521), 2) its septin-binding domain (residues 611–950) with a cryptic NLS mutated to alanine (pGF-IVL762; Finnigan et al., 2016), or 3) its septin-binding domain (611–950) fused to its C-terminal PtdSer-binding KA1 domain (residues 1245–1518; pGF-IVL536; Finnigan et al., 2016), each with eGFP fused to its C-terminus, were grown and visualized by fluorescence microscopy as in Figure 4A. Only cells with an intact septin collar were scored because, upon the onset of anaphase and formation of the split collar, Hsl1 is degraded (Burton and Solomon, 2000, 2001). (B) Diploids (176–190) expressing each of the three Hsl1 constructs in A that were N-terminally β10-(linker)32 tagged and each of the five mitotic septins C-terminally (linker)33-β11 tagged (left) visualized by fluorescence microscopy as in Figure 1B (right). For the 611–950 domain constructs (2 and 3) in these experiments, the cryptic NLS was mutated (R635A R636A K645A H648A K649A R653A K654A), as were both the KEN box (K775A E776A N777A) and D-box (R828A L831A) degradation motifs. (C) Quantification, as in Figure 1C, of the data shown in B, except that only budded cells with an intact septin collar (i.e., that had not entered cytokinesis) were scored. (D) Sixty diploids (186–190, 206–235, and 247–271) were generated in which the linker lengths in both the β10-tagged 611–950/1245–1518 fragment and in the septin-β11 constructs were systematically shortened, as indicated, visualized by fluorescence microscopy as in Figure 1B and quantified as in Figure 1C. Dashed green line, best fit trend line for the Cdc3 and Cdc12 data points; dashed blue line, best-fit trend line for Cdc10 data points.