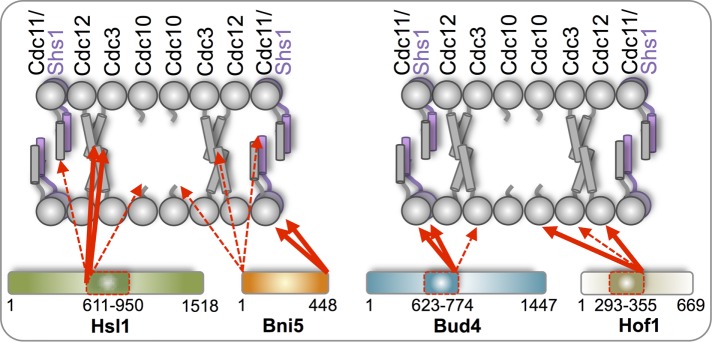
FIGURE 8:
Diagrammatic summary of the interactions of bud neck–localized proteins with specific septin subunits detected using the tripartite split-GFP assay. The linear hetero-octamer is depicted as it resides in paired filaments (conjoined via formation of cross-filament coiled coils between the CTEs on Cdc3 and Cdc12). The N-terminal face of each septin is predicted to project 180° away from its CTE, based on the recently determined crystal structure of S. cerevisiae Cdc11 as a representative subunit (Brausemann et al., 2016). The C-terminus of Bni5 exhibited preferential interaction with the N terminal faces of Cdc11 and Shs1 (thick red arrows), whereas the N- terminus of Bni5 was able to interact weakly with the C-termini of all five septins (dashed red arrows). The N-terminus of the septin-binding domain of Hsl1 had preferential interaction with the C-termini of Cdc12 and Cdc3, whereas the C-terminus of the same fragment did not have a detectable interaction with any subunit. The C-terminus of the septin-binding domain of Bud4 exhibited preferential interaction with the N-termini of Cdc11, Shs1, and, weakly, Cdc3 (most likely due to the extremely long N-terminal extension on Cdc3), whereas the N-terminus of the same fragment did not have a detectable interaction with any subunit. The C-terminus of the septin-binding domain of Hof1 had preferential interaction with the N-termini of Cdc10, Cdc12, and, weakly, Cdc3, whereas the N-terminus of the same fragment did not have detectable interaction with any subunit.