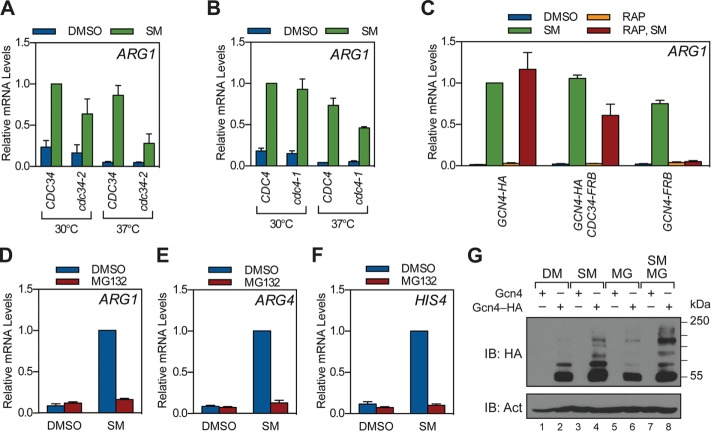
FIGURE 1:
Stimulation of Gcn4 target genes by SCFCdc4 and the proteasome. (A) CDC34 (W303-1a) and cdc34-2 (MT670) yeast were grown to log phase at 30°C in minimal medium and then shifted to 37°C or maintained at 30°C for 1 h as indicated. Strains were then treated with SM or DMSO for 1.5 h, at which time RNA was collected and ARG1 mRNA levels quantified by RT-qPCR. Relative mRNA levels for ARG1 were normalized to the CDC34 strain treated with SM at 30°C. n = 3. (B) As in A, except using CDC4 (W303-1a) and cdc4-1 (MT668) strains. n = 3. (C) Anchor-away strains expressing HA-tagged Gcn4 (GHY139), FRB-tagged Cdc34 (GHY149), or FRB-tagged Gcn4 (GHY145) were grown to log phase in minimal medium, treated with either DMSO or rapamycin for 1 h, and then further treated with either DMSO or SM for 1.5 h. RNA was collected, and ARG1 mRNA levels were measured by RT-qPCR, as in A. Relative mRNA levels for ARG1 were normalized to the GCN4-HA strain treated with SM at 30°C. n = 3. (D–F) Yeast bearing the pup1–T30A pre3–T20A mutations (GHY010) were grown to log phase in minimal medium and treated with either DMSO or MG132 for 1 h. Strains were then treated with SM or DMSO for 1.5 h, at which time RNA was collected and ARG1 (D), ARG4 (E), and HIS4 (F) mRNA levels quantified by RT-qPCR. Relative mRNA levels were then normalized to the SM-induced, DMSO-treated sample for each gene. n = 4. Error bars represent SEM. (G) Yeast expressing either native Gcn4 (GHY010) or HA-tagged Gcn4 (GHY025) were grown to log phase in minimal medium and treated with DMSO or MG132 for 1 h. Strains were then treated with SM or DMSO for 1.5 h, at which time protein was extracted, resolved by SDS–PAGE, and subject to Western blotting with antibodies against the HA epitope or β-actin (Act).