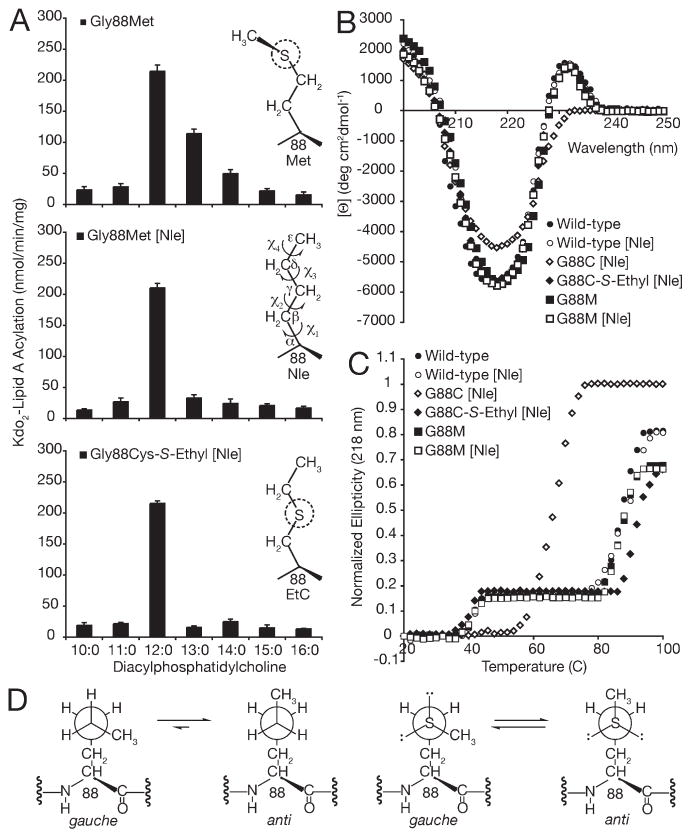
Figure 4.
Role of side chain flexibility and localized thioether–aromatic dispersion attraction in engineering a dedicated PagP lauroyltransferase. (A) PagP specific activity was measured using defined PtdCho’s varying in saturated acyl chain length fromC16 toC10. The unnatural amino acid Nle was substituted for Met by misaminoacylation of tRNAMet. Panels from top to bottom represent the activity profiles for Gly88Met, Gly88Met [Nle], and Gly88Cys-S-ethyl [Nle], respectively. For comparison, the behavior of Gly88Cys-S-ethyl PagP is shown in Figure 2. (B) Far-UVCD and (C) thermal melts at 218 nm reveal that substituting the five methionines in wild-type andGly88CysPagP (six inGly88MetPagP) with Nle does not compromise structure or stability. (D) Schematic representation of side chain conformational preferences for Nle (left) and Met (right). Viewed in Newman projection down the central χ3 torsion axis, the penultimate atom is rotated to emphasize the enthalpic preference of Nle for maintaining the terminalmethyl group in the extended anti conformation, where as Met is slightly favored to adopt one of the two available gauche conformers.