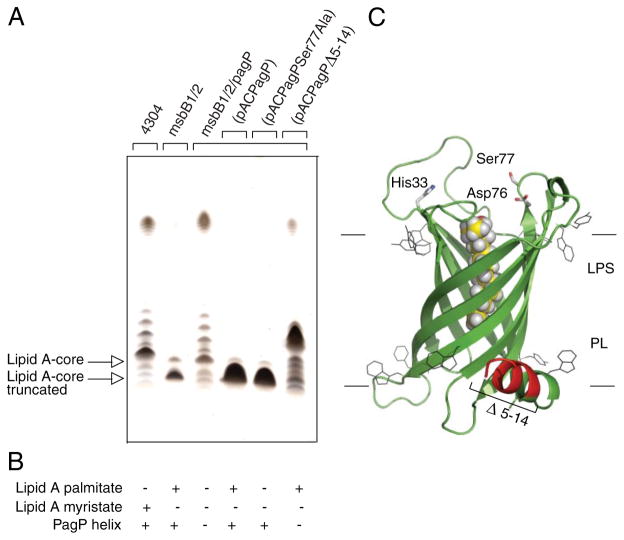
FIGURE 5. Complementation of the truncated R3 core oligosaccharide of msbB-deficient E. coli O157:H7.
Silver-stained 16% Tricine SDS-PAGE analysis of LPS isolated from wild-type E. coli O157:H7 strain 4304 and its lipid A acylation mutants deficient in either msbB or both msbB and pagP. Bacteria transformed with pACPagP, pACPagPSer77Ala, and pACPagPΔ5–14 are indicated (A). The presence (+) or absence (−) of the PagP periplasmic amphipathic α-helix and of lipid A acylated by palmitate or myristate, as deduced from Figs. 2 and 6, is indicated (B). Shown is a structural model of PagP derived from the crystal structure (Protein Data Bank code 1THQ). The first seven N-terminal residues are disordered in the crystal structure, but the approximate position of the Δ5–14 deletion in the periplasmic amphipathic α-helix (red) and the cell surface catalytic residues are indicated. The bound LDAO detergent molecule (yellow) identifies the hydrocarbon ruler. The aromatic belts define the boundaries of the OM, where LPS occupies the external leaflet and phospholipids (PL) occupy the periplasmic leaflet (C).