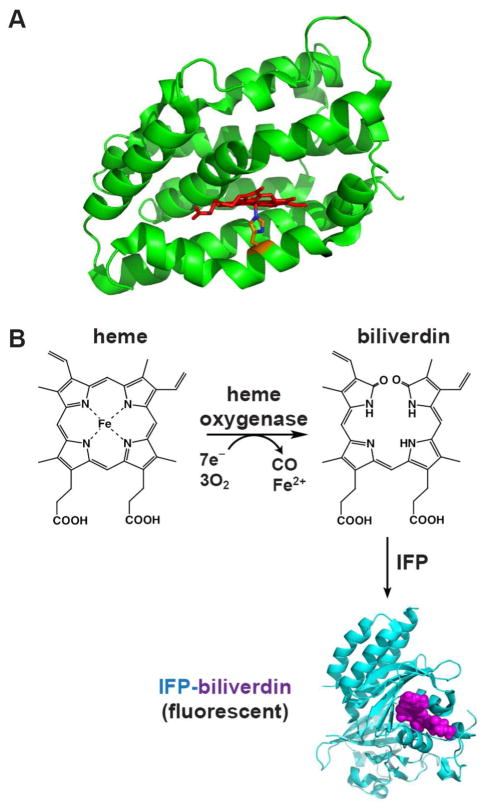
Figure 1. Heme oxygenase structure and reaction.
(A) Structure of human HO1 (PDB 1N45) highlighting the α-helical protein fold (green), bound heme (red), and proximal His (orange). (B) HO activity converts heme to biliverdin (BV), which can then be covalently bound by infrared fluorescent protein (IFP, cyan) to generate the fluorescent IFP-BV (bound BV in violet). The structure of IFP-BV was modeled using the BV-bound X-ray structure of the D. radiodurans chromophore-binding domain (PDB 3S7O), from which IFP is derived.