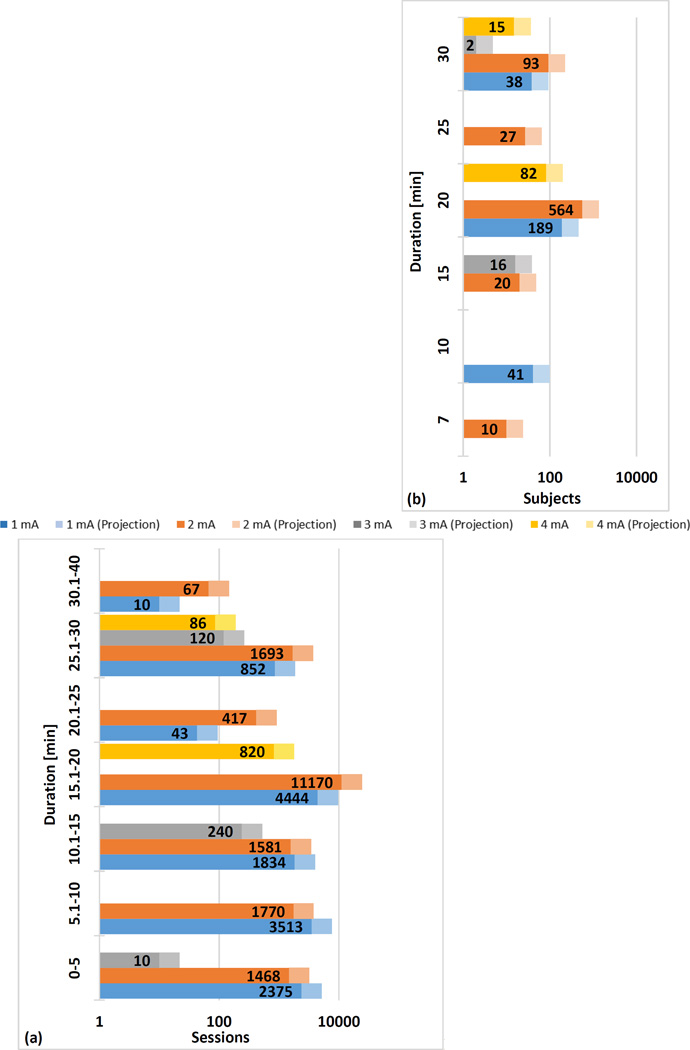
Figure 2. Number of Sessions by Duration (a) and Number of Subjects in Repeated Sessions by Duration (b).
Interim analysis of total sessions and dose in tDCS literature of published trials meeting our inclusion criterion. We searched the Pubmed database with the key words “transcranial direct current stimulation” limiting to papers published in English. We only included studies that met the following inclusion criteria: (1) used tDCS, (2) tested on human subjects, (3) reported original research, (4) used an electrolyte-soaked absorbent material, (5) clearly reported dosage information, and (6) published before July 2013. 488 of 1072 papers were considered. Of those, tDCS dosage and number of sessions were extracted. tDCS dosage includes current intensity, electrode size, duration, and position (not reported here). Number of sessions refers to the number of tDCS procedures completed (e.g. number of subjects times sessions per subject). If one subject underwent more than 4 sessions in one week, it was further classified as a repeated session. Data from ~55% of all original tDCS publications extracted using a combination of automated lexica analysis and manual screening (solid bar) and extrapolated based on update tDCS publication volume (lighter bar) – therefore the solid bars represent verified statistics until July 2013 while lighter bar represents prediction given fixed distribution of sessions in all papers to-date. We extracted the following dose parameters: Current applied, duration of session, current density at electrodes, and for studies with repeated sessions (three to seven treatments per week for at least one week) the number of sessions. If a clinical trial tested more than one tDCS condition per subject (e.g. anode vs. cathode over M1 separated by one week) these were considered separate sessions since the study design intended no carry-over effects and since the results of each session provide evidence for safety. From each qualified trial the total number of sessions and the parameters (intensity, duration, current density) for each session where determined. Therefore the total number of sessions applied at any given parameter (e.g. current of 2 mA) or combination of parameters (e.g. current of 2 mA and duration of 20 minutes) for tDCS trials is known. For repeated sessions, we aggregated data by number of subjects. Cumulative data is plotted under the assumption that increasing intensity or duration does not decrease risk, such that sessions at 2 mA support safety at 1 mA. This assumption presumes a monotonic dose-safety response curve and does not apply to efficacy.