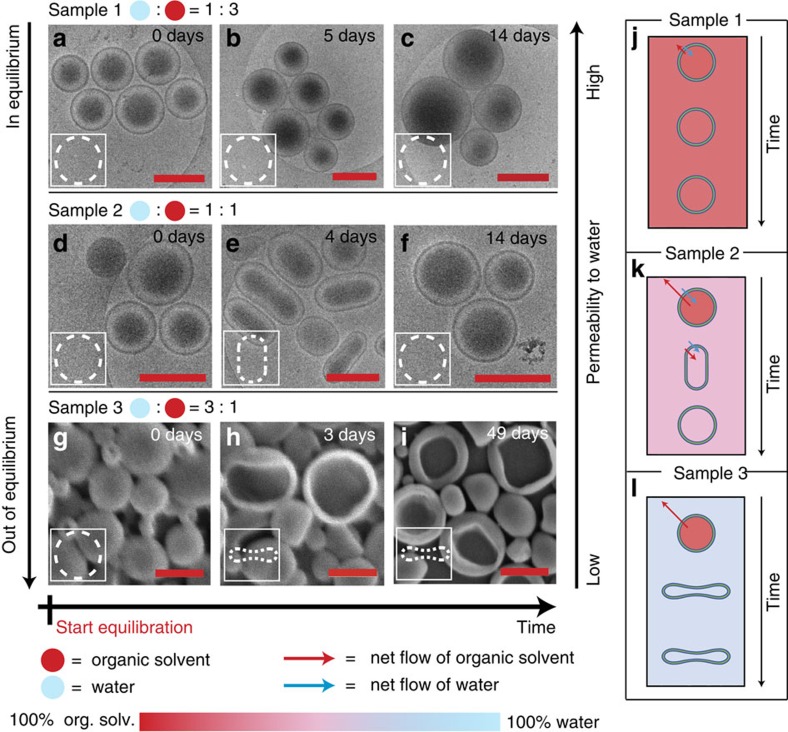
Figure 2. Polymersome morphology over time for different external solvent compositions.
(a–i) Electron microscopy images. All scale bars are 500 nm. (a–c) Cryo-TEM images of sample 1 (25% H2O and 75%THF/dioxane), showing no change in morphology over time. (d–f) Cryo-TEM images of sample 2 (50%H2O and 50%THF/dioxane), changing from spheres to a prolate morphology after 4 days and back to spheres after 14 days. (g–i) SEM images of sample 3 (75%H2O and 25%THF/dioxane) changing from spheres to discs after 3 days and still remaining discs after 49 days. The left arrow indicates the direction of increasing osmotic pressure; the right arrow indicates the direction of increasing permeability to water. (j–l) Schematic explanation. (j) Sample 1 is near osmotic equilibrium and the most permeable to water, allowing a simultaneous exchange of water and organic solvent to alleviate the small change in osmotic pressure without changing the shape. (k) Sample 2 is more out of equilibrium than sample 1 and less permeable to water. Organic solvent flows out faster than water flows in, causing a small deflation to form prolates. Subsequently, the bending energy is relieved by simultaneous inflow of water and organic solvent however at a much slower rate. (l) Sample 3 is the most out of osmotic equilibrium and impermeable to water. Therefore, these polymersomes deflate the most to form discs, and no reinflation is possible.