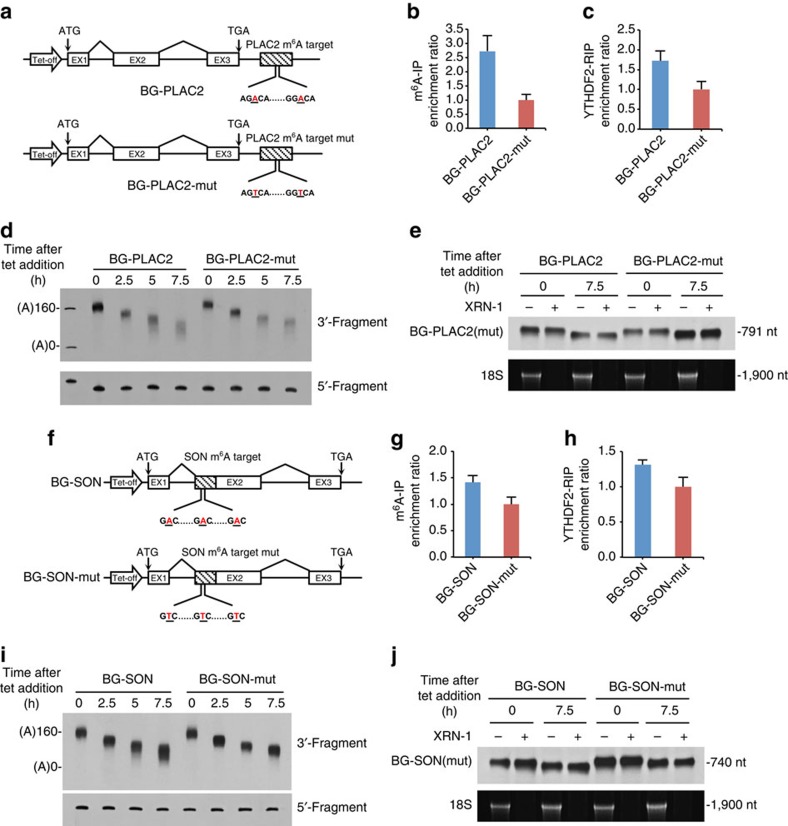
Figure 1. m6A modification promotes deadenylation of RNAs.
(a) The reporter constructs of BG-PLAC2 and BG-PLAC2-mut. A 135-nt fragment from lncRNA PLAC2 was inserted into the 3′-UTR of the BG reporter (BG-PLAC2). Blank box indicate the open reading frame (ORF). Striped box indicate the inserted DNA fragment. BG-PLAC2-mut is identical to BG-PLAC2, except that the two adenosines (underlined) within the m6A motifs were mutated to thymidines. (b) The m6A-IP enrichment ratio of BG-PLAC2 relative to BG-PLAC2-mut. (c) The YTHDF2-RIP enrichment ratio of BG-PLAC2 relative to BG-PLAC2-mut. The RIP assay was performed using anti-FLAG M2 antibody with HeLa-tTA cells that stably express FLAG-tagged YTHDF2. (d) Deadenylation assay of BG-PLAC2 and BG-PLAC2-mut. The brief removal of tetracycline (tet) from the culture medium generated a homogenous population of BG mRNAs that underwent synchronous decay. RNA samples were collected at the indicated time intervals and subjected to site-specific cleavage by RNase H to produce 3′- and 5′-BG mRNA fragments. RNA samples were then separated by electrophoresis and detected by northern blotting. (e) Retention of the 5′-cap on BG-PLAC2 and BG-PLAC2-mut undergoing deadenylation. The BG-PLAC2 and BG-PLAC2-mut RNA samples collected at 0 and 7.5 h from d were treated or not treated with the 5′-phosphate-dependent exonuclease XRN-1. 18S rRNA, which lacks a 5′-cap, served as a positive control for XRN-1 activity. (f–j) The same experiments as in a–e for BG-SON and BG-SON-mut. Error bars, mean±s.d., n=3, biological replicates.