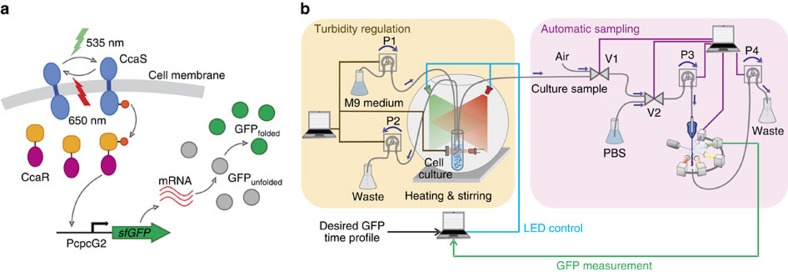
Figure 1. Light-switchable two-component system used in this work and experimental platform for optogenetic feedback.
(a) On absorption of green light, the sensor histidine kinase CcaS is quickly autophosphorylated and transfers its phosphate group to the cognate response regulator CcaR. Phosphorylated (active) CcaR in turn binds to the cpcG2 promoter to activate transcription of sfGFP. Absorption of red light inactivates CcaS, and transcription is eventually switched off. It has been hypothesized—but not yet conclusively demonstrated—that the inactive form of CcaS dephosphorylates CcaR. (b) Schematic of the constructed experimental platform containing the turbidity, autosampling and light-delivery modules. Straight lines denote control/measurement signals sent to/from the various devices. Curved lines indicate tubing segments. Arrows above tubing lines/pumps indicate the direction of flow/rotation. Computer icons are used to indicate control hardware and do not necessarily correspond to separate computing devices. Feedback control computations, LED control and autosampling are carried out by a single laptop, while turbidity control is coordinated by a programmable logic device. Further details are provided in the ‘Methods' section. Every 10 min., the sampling system acquires a culture sample via flow cytometry, saves and processes the sfGFP fluorescence data. On the basis of the measurement, the control algorithm determines the green-light-intensity level to be applied to the culture until the next measurement. In this way, the system can track a user-defined sfGFP expression profile.