Figure 1. INPP5E, an essential factor for autophagy.
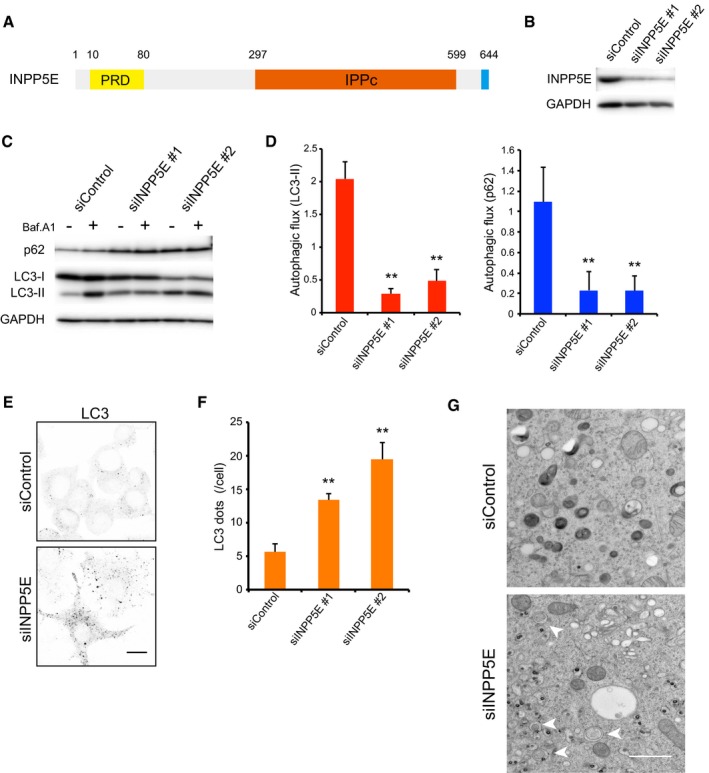
- Schematic diagram of human INPP5E. PRD, proline‐rich domain; IPPc, inositol polyphosphate phosphatase domain.
- Levels of INPP5E protein 72 h after transfection of N1E‐115 cells with control (siControl) or INPP5E‐specific siRNAs (siINPP5E #1 or #2), as determined by immunoblot.
- N1E‐115 cells treated with siControl or siINPP5Es were cultured for 2 h in growth medium with or without 125 nM bafilomycin A1 (Baf.A1) and then analyzed by immunoblot using anti‐p62, anti‐LC3, and anti‐GAPDH antibodies.
- Quantitation of protein signal intensities from immunoblots in (C) showing LC3‐II (left) or p62 (right) levels, following normalization to the control protein GAPDH. Because Baf.A1 inhibits autophagosome–lysosome fusion and degradation within lysosomes via inhibition of vacuolar‐type H+‐ATPase, it is possible to measure autophagic flux, that is, the degradation of autophagic substrates such as p62 and LC3‐II, by calculating the difference of the signal intensities of these proteins in the presence and absence of Baf.A1. Results represent the mean ± s.d. of three independent experiments. **P < 0.01.
- N1E‐115 cells treated with siControl or siINPP5Es were cultured in growth medium. Cells were fixed and stained with anti‐LC3 antibodies and analyzed by immunofluorescence microscopy. Scale bar, 10 μm.
- Quantitation of the number of LC3 puncta per cell as described in (E) (mean ± s.d.; n > 100 cells from three independent experiments). **P < 0.01.
- Electron micrographs in N1E‐115 cells treated with siControl or siINPP5Es were cultured in growth medium. Arrowheads indicate autophagosomes. Scale bar, 1 μm.
Source data are available online for this figure.
