Figure 1.
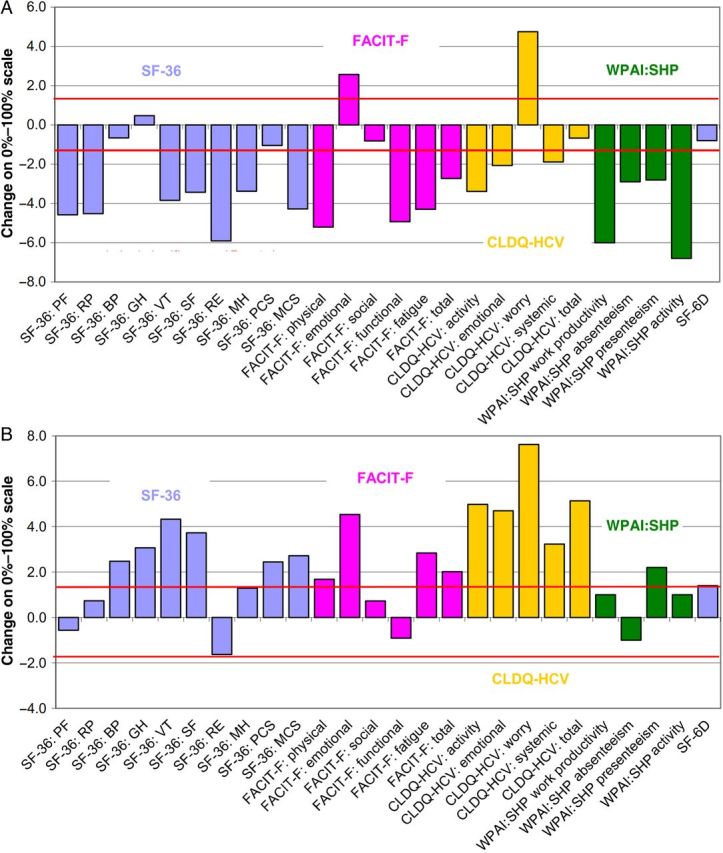
Changes in patient-reported outcomes at the end of treatment (A) and after sustaining a virologic response for 12 weeks after treatment cessation (B) in patients coinfected with human immunodeficiency virus and hepatitis C virus (HCV). Values above the upper and below the lower red lines denote statistically significant changes. Abbreviations: BP, bodily pain scale; CLDQ-HCV, Chronic Liver Disease Questionnaire–Hepatitis C Virus; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue questionnaire; MCS, mental component summary score; MH, mental health scale; PCS, physical component summary score; PF, physical functioning scale; RE, role emotional scale; RP, role physical scale; SF, social functioning scale; SF-36, Short-Form 36 questionnaire; VT, vitality scale; WPAI:SHP, Work Productivity and Activity–Specific Health Problem questionnaire.
