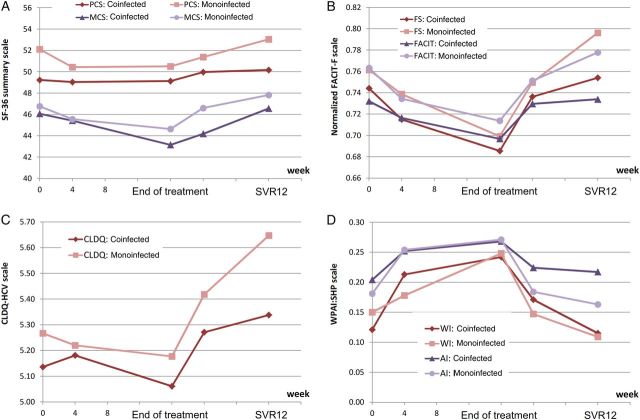
Figure 2.
Patient-reported outcomes throughout treatment with sofosbuvir/ribavirin in patients coinfected with human immunodeficiency virus and hepatitis C virus (HCV) and matched controls with HCV monoinfection. The physical component summary (PCS) score is significantly lower in HIV/HCV-coinfected patients, compared with HCV-monoinfected patients (P < .05) at baseline, 12 weeks after treatment cessation in those who achieved sustained virologic response (SVR12); mental component summary at posttreatment week 4; fatigue scale at SVR12; total Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) questionnaire baseline, SVR12; Chronic Liver Disease Questionnaire–Hepatitis C Virus (CLDQ-HCV) instrument at SVR12; work productivity and activity impairment of Work Productivity and Activity–Specific Health Problem (WPAI:SHP) questionnaire are similar between HIV/HCV-coinfected patients and HCV-monoinfected subjects at all time points (all P > .05). Abbreviations: AI, activity impairment; FS, fatigue scale; WI, work productivity impairment.