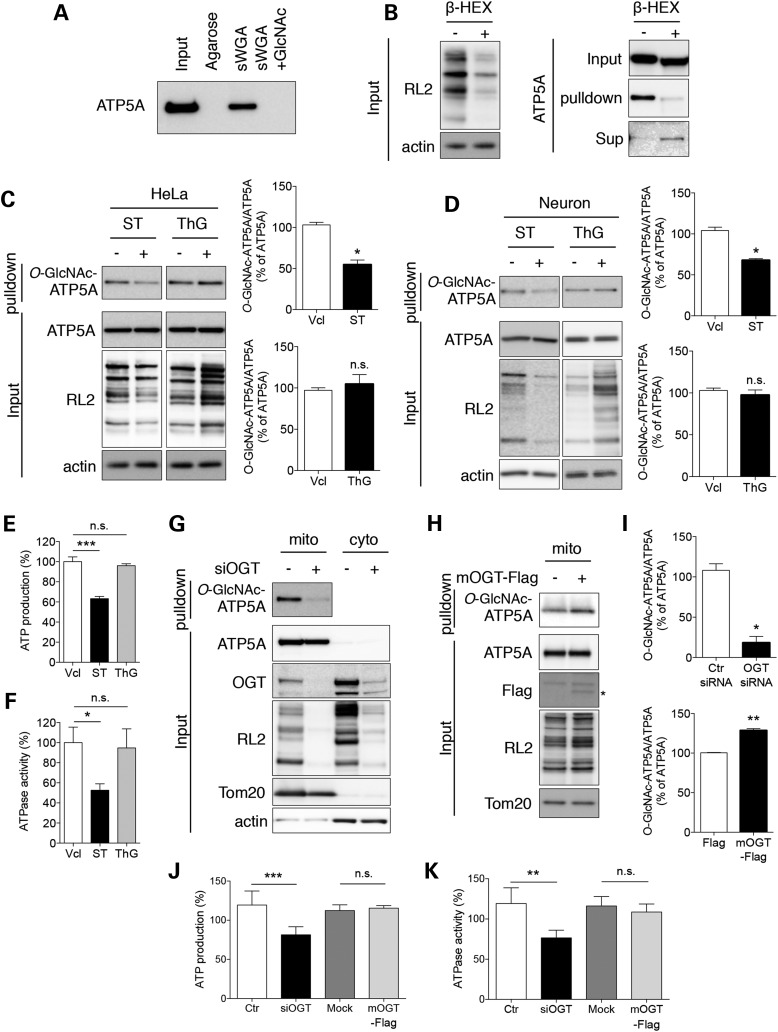
Figure 2.
O-GlcNAcylation of ATP5A in cultured cells. (A) Specificity of sWGA-agarose pull-down in a competition assay. All O-GlcNAcylated proteins were pulled down from total protein extracts (300 µg) of HeLa cells with agarose or sWGA-conjugated agarose in the presence or absence of free N-acetylglucosamine (GlcNAc) as a competitor. The level of ATP5A was measured by immunoblotting. (B) Specificity of sWGA-agarose pull-down by treatment with β-hexosaminidase (β-HEX). Total protein extracts (20 µg; left panel) or O-GlcNAc proteins pulled down with sWGA-agarose (right panel) were treated with β-HEX and analyzed for the total levels of O-GlcNAcylation (left panel) or O-GlcNAc-ATP5A in the indicated fractions (right panel) by western blotting. β-actin or total ATP5A were included as loading controls for each fraction. (C) Effect of changes of the O-GlcNAcylation machinery on O-GlcNAc-ATP5A in HeLa cells. HeLa cells were treated with an OGT inhibitor (ST045849; ST, 25 µM) or OGA inhibitor (Thiamet G; ThG, 1 µM) for 24 h. Samples or sWGA-agarose pull-down fractions were analyzed by western blotting with the indicated antibodies. Bar graphs show densitometric quantification of O-GlcNAcylated ATP5A in HeLa cells. (D) Effect of changes of the O-GlcNAcylation machinery on O-GlcNAc-ATP5A in primary cultured neurons. Primary cortical neurons were cultured from the brains of B6/SJL mice and treated as in (C). The level of O-GlcNAc-ATP5A was detected by western blotting. Bar graphs show densitometric quantification of O-GlcNAcylated ATP5A in neurons. (E, F) ATP production (E) and ATPase activity (F) from extracts of HeLa cell treated with OGA or OGT inhibitor. HeLa cells were incubated in the presence or absence of OGA or OGT inhibitor (1 µM ThG or 25 µM ST for 24 h). ATP production was measured as described in Materials and Methods (n = 3 per group). (G) Effect of OGT knockdown on O-GlcNAc-ATP5A in HeLa cells. HeLa cells were transfected with 20 nM siRNA against OGT. After 48 h, cells were fractionated and the mitochondrial and cytosolic fractions were analyzed by western blotting with the indicated antibodies. (H) Effect of mOGT overexpression on O-GlcNAc-ATP5A in HeLa cells. HeLa cells were transfected with a FLAG-mOGT construct. After 48 h, cells were fractionated and the mitochondrial fraction was analyzed by western blotting with the indicated antibodies. (I) Densitometric quantifications of O-GlcNAcylated ATP5A levels in OGT knockdown system (G) and mOGT overexpressing cells (H). (J, K) ATP production (J) and ATPase activity (K) from protein extracts of HeLa cells transfected with siRNA against OGT or FLAG-mOGT construct. ATP production and ATPase activity were measured as described in Materials and Methods (n = 3 per group). The results were represented as mean ± standard error from three independent experiments. ***P < 0.001; **P < 0.01; *P < 0.05; n.s.: non-significant.