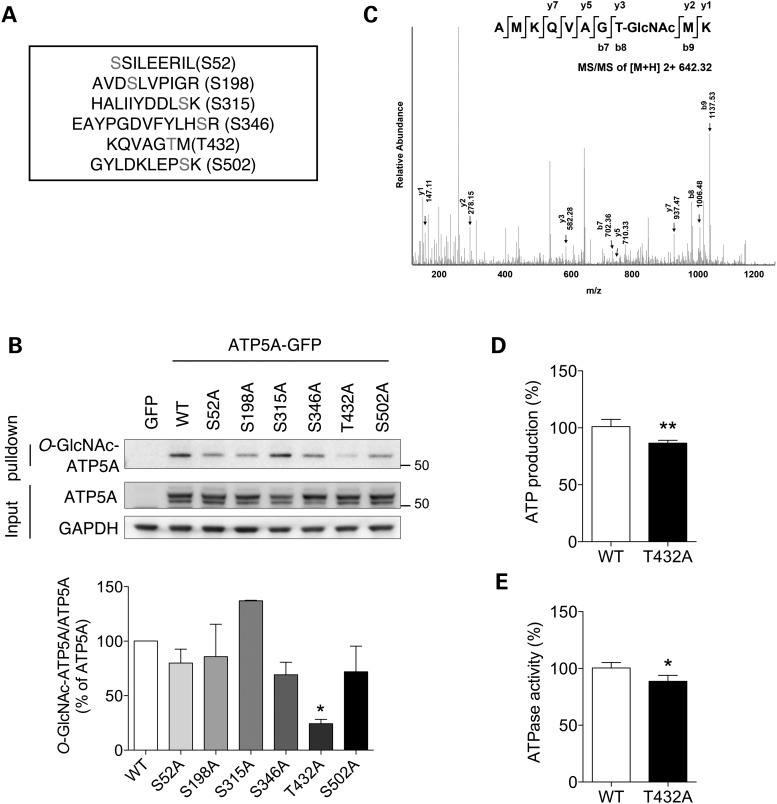
Figure 4.
Mapping of the O-GlcNAc modification sites on ATP5A. (A) LC-MS/MS result for putative O-GlcNAcylation sites on ATP5A. (B) Validation of O-GlcNAcylation on ATP5A through site-directed mutagenesis. Cells were transfected with GFP, WT ATP5A or mutant ATP5A in which putative O-GlcNAcylation sites were replaced with Ala. After 48 h transfection, whole cell lysates were prepared and pulled down with sWGA-agarose beads. O-GlcNAcylated ATP5A was detected by western blotting. Bar graph shows densitometric quantification of O-GlcNAcylated ATP5A in cells. (C) O-GlcNAcylation of ATP5A on Thr432. (D, E) ATP production (D) and ATPase activity (E) from extracts of HeLa cell transfected with ATP5A WT or ATP5A T432 mutant construct (T432A). After 48 h, ATP production and ATPase activity were measured as described in Materials and Methods (n = 3 per group). The results were represented as mean ± standard error from three independent experiments. **P < 0.01; *P < 0.05; n.s.: non-significant.