Abstract
Primary immunodeficiency disorders (PIDDs) are a heterogeneous group of inherited disorders of the immune system. Currently more than 120 different PIDs with a known genetic defect have been recognized. The diagnosis of many of these disorders is supported strongly by a wide variety of flow cytometry applications. Flow cytometry offers a rapid and sensitive tool for diagnosis and classification of PIDs. It is applicable in the initial workup and subsequent management of several primary immunodeficiency diseases. As our understanding of the pathogenesis and management of these diseases increases, majority of these tests can be easily established in diagnostic laboratory. Thus, the focus of this article is on the application of flow cytometry in the diagnosis and/or evaluation of PIDDs.
Keywords: Primary immunodeficiency, flowcytometry, diagnosis
The evaluation of a patient with a possible primary immunodeficiency disorder (PIDD) routinely includes the use of flow cytometry directed at the assessment for specific cell populations plus the presence of specific cell surface and intracellular proteins. More recently, techniques using flow cytometry have been applied to assess functional characteristics that are linked to PIDD [1]. The focus of this article is on the application of flow cytometry in the diagnosis and/or evaluation of PIDDs.
Evaluation of PIDD Associated with Altered Lymphocyte Populations (or Subpopulations)
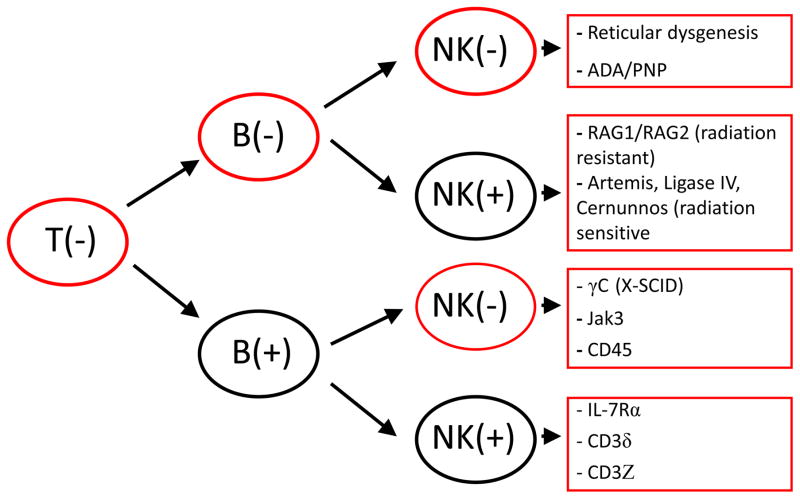
Selected PIDDs are characterized by decrease in one of more lymphocyte populations. An initial screening approach involves determination of the absolute lymphocyte count (T, B and NK cells) and comparison with age matched reference (control) data since there is a marked change in lymphocyte counts linked to age [2]. This is particularly relevant for any defect that blocks T cell development based on the fact that T cells constitute 60–75% of the circulating lymphocytes, thus any disorder that interferes with T cell development would be expected to result in lymphocytopenia. In the profound immune defect severe combined immunodeficiency (SCID), lymphocytopenia is expected to be present since the common feature in all of these patients is a block in T cell development [3]. However; there are exceptions to this in the setting of hypomorphic mutations in a SCID causing genes or in the case of significant maternal T cell engraftment. Thus the immunophenotype in typical SCID is characterized by absence or marked decrease in T cells while the impact on B cells and NK cell numbers depends on the specific genetic defect underlying the disease [3]. Immunophenotyping in the setting of a potential SCID diagnosis has resulted in four general disease categories defined at the impact on lymphocyte populations: T−/B−/NK−, T−/B−/NK+, T−/B+/NK−, and T−/B+/NK+SCID (Figure1) [4–5]. Overall, patients with ADA deficiency and reticular dysgenesis demonstrate the T−/B−/NK− immunophenotype and typically have the most profound lymphocytopenia among SCID patients [6]. Newborn screening for SCID is currently being performed in most states within the United States. This is based on the T cell receptor excision circle (TREC) assay and has clearly established that approximately 20% of SCID patients identified using this unbiased approach have hypomorphic mutations [7]. This type of presentation is typically associated with a more varied clinical and laboratory phenotype including lymphocyte numbers that are usually significantly higher than seen in typical SCID.3 In addition, both hypomorphic SCID mutations and maternal engraftment (found only in the setting of patients with typical SCID) result in circulating T cells that characteristically are not naïve cells (CD45 RA−, CD45 RO+). This contrasts with the expected predominance of naïve T cells that is normally seen in healthy newborns. In addition, the circulating cells in the setting of either hypomorphic SCID mutations or maternal engraftment often express HLA-DR.
Figure 1.
immunophenotypic characteristics of SCID based on T, B and NK cell distribution with associated defects. [PNP: Purine nucleoside phosphorylase; ADA: Adenosine deaminase deficiency].
The major advantage to the early detection of SCID is that it facilitates the prompt initiation of appropriate immune reconstitution therapy at a time when the overall outcome of therapy is optimal [8–9]. Early diagnosis also allows appropriate patient management in terms of blood products(if required, should be filtered for white blood cells and irradiated) in order to prevent the potential for transfusion based graft versus host disease (GVHD) as well as preventing the administration of live vaccines [10].
Patients with markedly decreased to absent T lymphocytes, but normal B and NK cell numbers, could also have complete DiGeorge syndrome [11–12]. Typically the distinction between T−/B+/NK+SCID and DiGeorge syndrome is obvious owing to the additional findings in the latter disorder including craniofacial abnormalities, hypocalcemia (often with tetany) due to hypoparathyroidism and/or conotruncal cardiac disease. In the setting of either diagnosis further workup would include assessment for the presence of circulating naïve T cells by evaluating CD 45RA expression often together with either CD31 or CD62L.
Elevation of double negative DNT cells that express the alpha beta+ T cell receptor in peripheral blood and lymphoid tissues is suggestive of the autoimmune lymphoproliferative syndrome (ALPS). It has been recommended that the percentage of T cell receptor (TCR) alpha beta+ Double Negative T cells (DNT cells) required for a diagnosis of ALPS is greater than or equal to 1.5% of total lymphocytes or 2.5% of T lymphocytes, in the setting of normal or elevated lymphocyte counts [13]. However, presence of lymphopenia invalidates this criterion. DNT cells in ALPS patients appear to be a homogeneous population of cells expressing CD45RA, CD57, CD27, CD28, perforin and HLA-DR, but lacking CD45RO and CD56 [14]. This is in contrast with the heterogeneous population of DNTs found in the peripheral blood of healthy controls that predominantly express the gamma delta form of the T cell receptor.
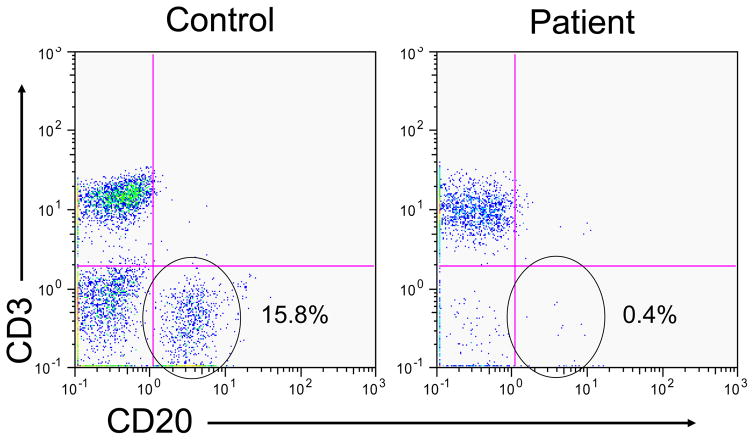
Patients with decreased immunoglobulin levels should be evaluated for B cell numbers and if low (<2%) during infancy or early childhood, congenital agammaglobulinemia either X-lined (XLA) or autosomal recessive should be suspected (Figure 2). The definitive diagnosis in this setting would typically depend on defining a genetic mutation associated with agammaglobulinemia and B cell lymphopenia [4, 15]. B cell quantitation can still be useful in patients who present with hypogammaglobulinemia at an older age, but it generally has less direct impact on defining the diagnosis. More recently, later onset hypogammagloblulinemia, in particular common variable immunodeficiency (CVID), has been found to be linked to alterations in B cell subpopulations, particularly affecting memory B cell subpopulations (CD27+) [16]. Regardless of the B cell status in the peripheral blood, establishing a diagnosis of antibody deficiency via quantitative immunoglobulin levels and responses to vaccines is critical in these patients to initiate replacement immunoglobulin replacement therapy to prevent destructive lung changes associated with recurrent and/or chronic bacterial infection.
Figure 2.
flow cytometric dot plot of a control subject and a patient with congenital X-linked agammaglobulinemia demonstrating marked decrease in circulating B cells
There has been recognition that selected PIDDs may be linked to NK cell defects. Among these patients are individuals who lack circulating NK cells as defined by the absences of CD3−/CD56+ cells [17]. The clinical history in this setting typically is associated with increased susceptibility to herpes virus infections and currently there are at least two genetic defects associated with NK cell deficiency (autosomal dominant GATA2 and autosomal recessive MCM4 defects). There are also functional NK cell abnormalities and selected defects of NK cell function are discussed in a later section focused on cell function analysis by flow.
Evaluation of PIDD to Detect Defects in Cell Surface Protein Expression
Flow cytometry can be useful in the diagnosis of immuno-deficiencies associated with defects in the expression of cell surface proteins. In X-linked hyper IgM syndrome, CD4+ T lymphocytes can be studied for the expression of CD40 ligand (CD154) following activation [18]. Other PIDD examples where surface protein expression could prove useful in the clinical evaluation include SCID associated with a defect in the expression of the IL7R alpha chain and specific CD3 chains. Additionally in combined immunodeficiencies with specific clinical phenotypes evaluation of Major histocompatibility complex (MHC) class I and class II expression can be a useful screening technique [4].
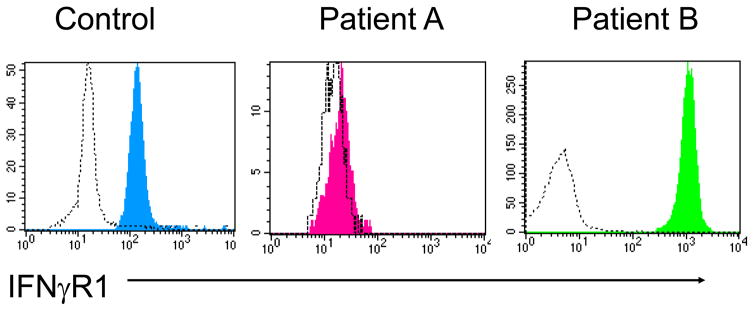
Flow cytometry is also useful in evaluating for the expression of interferon gamma receptor 1onmonocytes in the setting of suspected Mendelian susceptibility to mycobacterial disease (MSMD). A subgroup of these patients can have either an autosomal recessive (absent receptor expression) or an autosomal dominant (increased receptor expression) defects in this protein that can be differentiated by flow cytometry (Figure 3) [18]. Within MSMD patients, the most commonly found defect affects the IL-12 receptor β1 chain and this also can be evaluated by flow cytometry but only following T cell activation as this protein is not expressed constitutively by T cells [19].
Figure 3.
Flow cytometric histograms demonstrating the expression of IFNγR on monocytes from a control subject, a patient with an autosomal recessive IFNγR1 defect (Patient A) and a patient with an autosomal dominant IFNγR1 defect (Patient B).
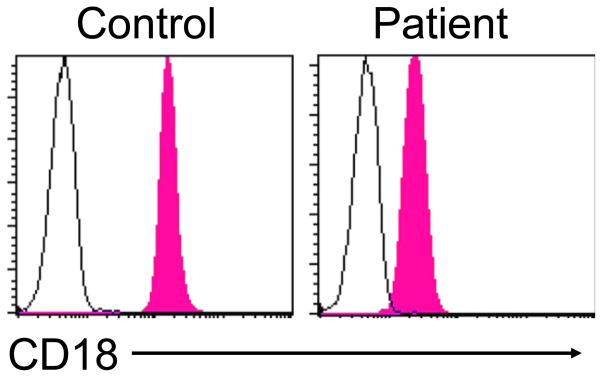
Leukocyte adhesion deficiency (LAD) type 1 associated with recurrent bacterial infections due to a defect in β2 integrin expression can be diagnosed by evaluating for the presence of cell surface CD18 (Figure 4, one also can test for CD11a, b, c expression) [20]. In patients with LAD1, CD18 is usually less than 5–10% of normal and unlike controls the level of expression is not upregulated following neutrophil activation. Finally, in LAD type 2 associated with defective fucosylation, the diagnosis can be inferred by demonstrating failure of CD15s (Sialyl Lewis X antigen) expression together with red blood cell typing that yields the rare Bombay blood type [20]. The caveat in all of these surface protein evaluations is that flow cytometric testing does not assess function. Therefore, as is the case with any immunoassay testing, protein absence proves to be diagnostic but protein presence reflects either disease due to a dysfunctional protein or a normal state. Resolution of this situation would require moving to a testing approach that evaluates protein function.
Figure 4.
Flow cytometric histograms from a control subject and a patient with leukocyte adhesion deficiency (LAD) type1 demonstrating markedly diminished CD18 expression on neutrophils
Evaluation of PIDD to Detect Defective Intracellular Protein Expression
There are a number of PIDDs associated with defective intracellular protein expression and in selected disorders this can be an effective screening test using flow cytometry. However, as is the case with cell surface expression, the same caveat applies, namely absence of protein expression is diagnostic but presence of protein is not. In Wiskott Aldrich syndrome (WAS) and X linked thrombocytopenia (XLT), absence or decreased expression of the Wiskott Aldrich protein (WASP) can help establish the diagnosis. However, presence of normal intracellular WASP expression by flow cytometry does not rule out the diagnosis since some patients (particularly those with XLT) express a dysfunctional but detectable protein at levels comparable to that found in controls [21]. As mentioned above, in patients with X linked agammaglobulinemia, a monoclonal antibody to detect BTK can be used as a screening test but the target should be either monocytes or platelets since the patients lack B cells [22].
Laboratory screening for immune dysregulation, polyendo-crinopathy, enteropathy, X-linked (IPEX) is typically performed by evaluating CD4+ T cells for surface expression of CD25 together with intracellular expression of FOXP3. The diagnosis of IPEX is confirmed in a male patient whose CD4+ T cells demonstrate absence of FOXP3 expression [23]. In DOCK8 deficiency evaluating for intracellular DOCK8 expression demonstrates absent or diminished levels of protein in many of these patients (personal observation, TAF and SDR). Finally, in patients with familial hemophagocytic lymphohistiocytosis (FLH), intracellular slam associated protein (SAP), x-linked inhibitor of apoptosis (XIAP) and perforin expression can be evaluated to help screen for XLP1, XLP2 or FLH type 2, respectively [24].
Evaluation of PIDD Using Flow Cytometry to Detect Functional Abnormalities
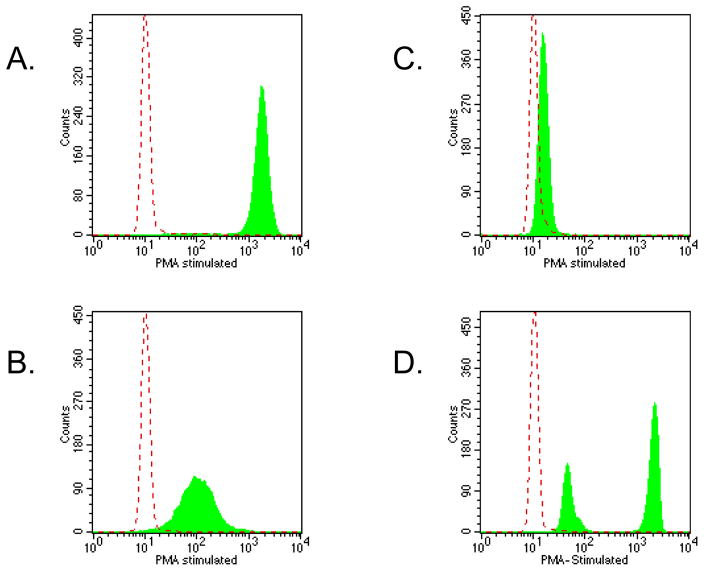
More recently, flow cytometry has been used to evaluate specific functional features and this has evolved into applications useful in the diagnosis of PIDD. The most commonly used functional assay is the evaluation of oxidative burst following granulocyte activation using the dihydrorhodamine 123 (DHR) assay (Figure 5) [25]. Following cell activation with phorbol myristate acetate (PMA), DHR loaded granulocytes from a control individual demonstrate a marked (>100 fold) increase in fluorescence. In contrast, granulocytes from chronic granulomatous disease (CGD) patients are defective in NADPH oxidase function and these results in absent or markedly diminished oxidative burst reflected by markedly diminished fluorescence following PMA activation. The degree of the oxidative defect assessed using the DHR flow assay has been correlated with patient outcome [26]. This assay is also useful in evaluating for carrier status in female relatives of patients with X-linked CGD.
Figure 5.
DHR flow cytometry assay histograms demonstrating the change (or lack thereof) in fluorescence in neutrophils following PMA stimulation in a control subject (panel A), a patient with autosomal recessive (p47 phox) CGD (panel B), a patient with X-linked recessive (gp91 phox) recessive CGD (panel C) and a X-linked carrier (panel D). The dashed line shows the background fluorescence of unstimulated neutrophils
Defects associated with FLH generally are associated with abnormal NK cell function and this can be evaluated using a flow cytometry assay that evaluates the expression of CD107a on NK cells. This protein is normally expressed on NK cells following incubation with specific target cells (e.g. K562 cells) [23, 27]. In patients with interferon gamma receptor 1 or receptor 2 deficiency, the expected phosphorylation of STAT1 is absent after the addition of interferon gamma. Historically, this was evaluated by Western blot but it has been shown that this also can be assessed using flow cytometry with a reagent specific for the phosphorylated form of STAT1 [28]. Likewise within the group of patients with MSMD, IL-12 receptor defects can be evaluated functionally by assessing for STAT4 phosphorylation following the addition of IL-12 to pre-activated T cells (in order to upregulate the normal expression of the IL-12 receptor) [29]. Additional assays to evaluate the phosphorylation of other intracellular proteins have been developed that may be useful in assessing signaling defects associated with certain PIDD.
Evaluation of Disorders with Decreased Lymphocytes not due to PIDD
The unexpected finding of a decreased absolute lymphocyte counts should provoke careful scrutiny of the complete blood count, differential and cell morphology. In previously healthy individuals, repeating the CBC and differential would be a reasonable first step to ensure the finding is not related to a technical problem. If lymphocytopenia persists, further immune workup would be prudent and lymphocytopenia associated with opportunistic infection should result in evaluation of serologies/viral load for HIV. Alternatively, significant lymphopenia can also be associated with lymphatic loss such as is found in intestinal lymphangiectasia and clues to this diagnosis would include the presence of hypogammagolulinemia and hypoalbuminemia.
When any immune evaluation involves flow cytometry, the data must include both percentage and absolute numbers. In addition, it is critical to compare patient results with appropriate age matched reference data. Furthermore, a simple validity check for flow cytometry data consists of assessing the sum of the percentage of T cells, B cells and NK cells that should equal approximately 100% unless there are atypical lymphocytes (e.g. leukemic cells) or a technical problem. Finally, control data is typically expressed as a 95% confidence interval meaning that2.5% of “normals” fall below and above this interval.
Conclusion
The use of flow cytometry has emerged as a very useful laboratory tool in evaluating patients with potential PIDDs. In some cases, this methodology can serve as a laboratory technique to help establish a specific diagnosis while in others it provides useful information regarding the underlying immunologic defect.
Acknowledgments
Source of Support: This work was supported by the Intramural Research Program of the NIH Clinical Center, National Institutes of Health, Bethesda, MD, USA
Footnotes
Disclaimers: Nil
Declaration on competing interests: Nil
References
- 1.Oliveira JB, Fleisher TA. Molecular- and flow cytometry-based diagnosis of primary immunodeficiency disorders. Curr Allergy Asthma Rep. 2010;10(6):460–67. doi: 10.1007/s11882-010-0137-8. [DOI] [PubMed] [Google Scholar]
- 2.Shearer WT, Rosenblatt HM, Gelman RS, et al. Pediatric AIDS Clinical Trials Group. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112(5):973–80. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Shearer WT, Dunn E, Notarangelo LD, et al. Establishing the diagnostic criteria for serve combined immunodeficiency disease (SCID), leaky SCID and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol. 2014;133(4):1092–98. doi: 10.1016/j.jaci.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Herz W, Bousfiha A, Casanova JL, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol. 2014;5:162. doi: 10.3389/fimmu.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalman L, Lindegren ML, Kobrynski L, Vogt R, Hannon H, Howard JT, Buckley R. Mutations in genes required for T-cell development: IL7R, CD45, IL2RG, JAK3, RAG1, RAG2, ARTEMIS, and ADA and severe combined immunodeficiency: HuGE review. Genet Med. 2004;6(1):16–26. doi: 10.1097/01.GIM.0000105752.80592.A3. [DOI] [PubMed] [Google Scholar]
- 6.Villa A, Sobacchi C, Notarangelo LD, Bozzi F, et al. V(D)J recombination defects in lymphocytes due to RAG mutations: severe immunodeficiency with a spectrum of clinical presentations. Blood. 2001;97(1):81–8. doi: 10.1182/blood.v97.1.81. [DOI] [PubMed] [Google Scholar]
- 7.Stephan JL, Vlekova V, Le Deist F, et al. Severe combined immunodeficiency: a retrospective single-center study of clinical presentation and outcome in 117 patients. J Pediatr. 1993;123(4):564–72. doi: 10.1016/s0022-3476(05)80951-5. [DOI] [PubMed] [Google Scholar]
- 8.Kwan A, Abraham RS, Currier R, et al. Newborn screening for severe combined immunodeficiency in 11 screen programs in the United States. JAMA. 2014;312(7):729–38. doi: 10.1001/jama.2014.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pai SY, Logan B, Griffith L, et al. Transplantation outcomes of severe combined immunodeficiency, 2000–2009. N Engl J Med. 2014;371(5):434–46. doi: 10.1056/NEJMoa1401177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99(3):872–8. doi: 10.1182/blood.v99.3.872. [DOI] [PubMed] [Google Scholar]
- 11.Medical Advisory Committee of the Immune Deficiency Foundation. Shearer WT, Fleisher TA, Conley ME, et al. Recommendations for live viral and bacterial vaccines in immunodeficient patients and the close contacts. J Allergy Clin Immunol. 2014;133(4):961–6. doi: 10.1016/j.jaci.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markert ML, Hummell DS, Rosenblatt HM, et al. Complete DiGeorge syndrome: persistence of profound immunodeficiency. J Pediatr. 1998;132(1):15–21. doi: 10.1016/s0022-3476(98)70478-0. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira JB, Bleesing JJ, Dianzani U, et al. Revised diagnostic criterial and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010;116(14):e35–40. doi: 10.1182/blood-2010-04-280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chinen J, Rosenblatt HM, Smith EO, Shearer WT, Noroski LM. Long-term assessment of T-cell populations in DiGeorge syndrome. J Allergy Clin Immunol. 2003;111(3):573–9. doi: 10.1067/mai.2003.165. [DOI] [PubMed] [Google Scholar]
- 15.Conley ME. Genetics of hypogammaglobulinemia: what do we really know. Curr Opin Immunol. 2009;21(5):466–71. doi: 10.1016/j.coi.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warnatz K, Schlesier MA. Flow cytometric phenotyping of common variable immunodeficiency. Cytometry B Clin Cytom. 2008;74(5):261–271. doi: 10.1002/cyto.b.20432. [DOI] [PubMed] [Google Scholar]
- 17.Orange JS. NK cell deficiency. J Allergy Clin Immunol. 2013;132(3):515–25. doi: 10.1016/j.jaci.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freyer DR, Gowans LK, Warzynski M, Lee WI. Flow cytometric diagnosis of X-linked hyper-IgM syndrome: application of an accurate and convenient procedure. J Pediatr Hematol Oncol. 2004;26(6):363–370. doi: 10.1097/00043426-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Boisson-Dupuis JS, Abel L, Casanova JL. Mendelian susceptibility to mycobacterial disease: genetic, immunological and clinical features of inborn errors of IFN-γ immunity. Seminar Immunol. 2014;26(6):454–70. doi: 10.1016/j.smim.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van de Vijver E, van den Berg TK, Kuijpers TW. Leukocyte adhesion deficiencies. Hematol Oncol Clin North Am. 2013;27(1):101–16. doi: 10.1016/j.hoc.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima M, Yamada M, Yamaguchi K, et al. Possible application of flow cytometry for the evaluation of the structure and functional status of WASP in peripheral blood mononuclear cells. Eur J Haematol. 2009;82(3):223–30. doi: 10.1111/j.1600-0609.2008.01180.x. [DOI] [PubMed] [Google Scholar]
- 22.Kanegane H, Futatani T, Wang Y, et al. Clinical and mutational characteristics of X-linked agammaglobulinemia and its carrier identified by flow cytometric assessment combined with genetic analysis. J Allergy Clin Immunol. 2001;108(6):1012–20. doi: 10.1067/mai.2001.120133. [DOI] [PubMed] [Google Scholar]
- 23.d’Hennezel E, Bin Dhuban K, Torgerson T, Pinccinrillo CA. The immungenetics of immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) J Med Genet. 2012;49(2):291–302. doi: 10.1136/jmedgenet-2012-100759. [DOI] [PubMed] [Google Scholar]
- 24.Johnson TS, Vilaneuva J, Filipovich AH, Marsh RA, Bleesing JJ. Contemporary diagnostic methods for hemophagocytic lymphohistiocytic disorders. J Immunol Method. 2011;364(1–2):1–13. doi: 10.1016/j.jim.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Jirapongsananuruk O, Malech HL, Kuhns DB, Niemela JE, Brown MR, Anderson-Cohen M, Fleisher TA. Diagnostic paradigm for evaluation of male patients with chronic granulomatous disease, based on the dihydrorhodamine 123 assay. J Allergy Clin Immunol. 2003;111(2):374–9. doi: 10.1067/mai.2003.58. [DOI] [PubMed] [Google Scholar]
- 26.Kuhns DB, Lavord WG, Heller T. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med. 2010;363(27):2600–2610. doi: 10.1056/NEJMoa1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcenaro S, Gallo F, Martini S, et al. Analysis of natural killer-cell function in familial hemophagocytic lymphohistiocytosis (FHL): defective CD107a surface expression heralds Munc13–4 defect and discriminates between genetic subtypes of the disease. Blood. 2006;108(7):2316–23. doi: 10.1182/blood-2006-04-015693. [DOI] [PubMed] [Google Scholar]
- 28.Fleisher TA, Dorman SE, Anderson JA, Vail M, Brown MR, Holland SM. Detection of intracellular phosphorylated STAT-1 by flow cytometry. Clin Immunol. 1999;90(3):425–30. doi: 10.1006/clim.1998.4654. [DOI] [PubMed] [Google Scholar]
- 29.Uzel G, Frucht DM, Fleisher TA, Holland SM. Detection of intracellular phosphorylated STAT4 by flow cytometry. Clin Immunol. 2001;100(3):270–76. doi: 10.1006/clim.2001.5078. [DOI] [PubMed] [Google Scholar]