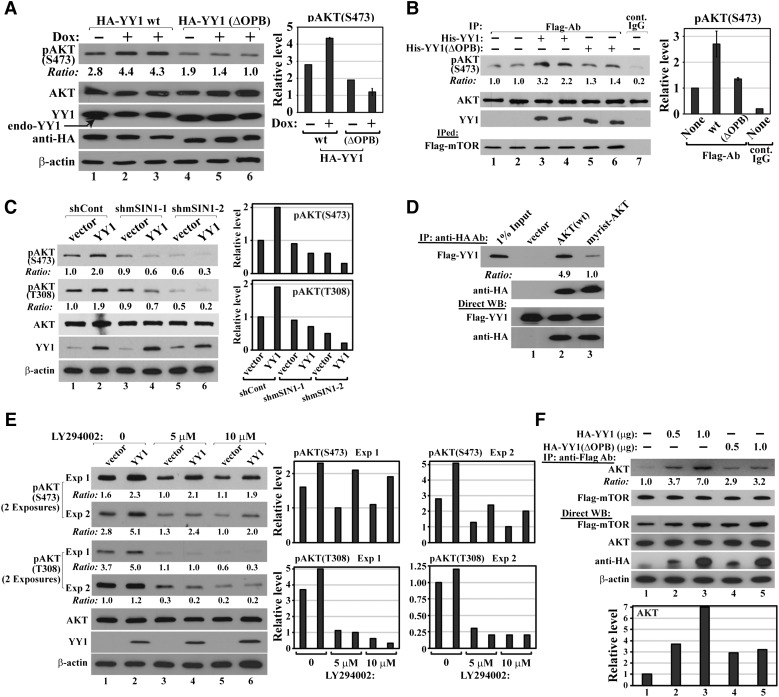
Figure 4.
YY1 binding promotes AKT phosphorylation. (A) YY1(wt) but not YY1(ΔOPB) increases AKT phosphorylation. MDA-MB-231 cells with Dox-inducible shYY1-2 were infected by lentiviruses generated with pSL5/HA-YY1 wt or ΔOPB, and cultured in the absence or presence of Dox. Cell lysates were analyzed by western blot using the indicated antibodies. Duplicated samples: 2–3 and 5–6. (B) His-YY1 wt, but not ΔOPB, markedly enhances mTOR-mediated pAKT(S473) in vitro. Flag-mTOR was IPed in a CHAPS-containing buffer from HeLa cells transfected with pcDNA3/Flag-mTOR and incubated with recombinant GST-AKT and His-YY1(wt) or His-YY1(ΔOPB). Duplicated samples: 1–2, 3–4, and 5–6. Samples were analyzed for pAKT(S473), AKT, YY1, and Flag-mTOR. (C) mSIN1 knockdown blocks YY1-promoted AKT phosphorylation. Nontumorigenic 184B5 cells were infected by lentivirus carrying control or mSIN1 shRNAs. Five days post infection and puromycin selection, cell lysates were analyzed by the indicated antibodies. (D) AKT myristoylation decreases its interaction with YY1. Myrist, myristoylation. Constructs of empty vector, HA-AKT(wt), or HA-myrist-AKT were co-transfected into HeLa cells with Flag-YY1-expressing plasmid. HA antibody-conjugated agarose was used in co-IP assays with the indicated antibodies. (E) YY1 increases AKT phosphorylation in the presence of PI3K inhibitor. 184B5 cells infected by lentivirus carrying an empty vector or expressing YY1 were treated with two different concentrations of LY294002 for 16 h. Cell lysates were analyzed by the indicated antibodies. (F) YY1 enhances AKT and mTOR interaction. HA-YY1(wt) or HA-YY1(ΔOPB) plasmid was co-transfected with Flag-mTOR and AKT constructs. Flag antibody-conjugated agarose beads were used in co-IP studies with the indicated antibodies. In A, B, C, E, and F, densitometry analyses of western blots were shown at the right.