Abstract
With the introduction and establishment of exotic species, most ecosystems now contain both native and exotic plants and herbivores. Recent research identifies several factors that govern how specialist herbivores switch host plants upon introduction. Predicting the feeding ecology and impacts of introduced generalist species, however, remains difficult. Here, we examine how plant geographic origin, an indicator of shared co-evolutionary history, influences patterns of host use by a generalist, invasive herbivore, while accounting for variation in plant availability. The brown marmorated stink bug, Halyomorpha halys, is a highly polyphagous Asian herbivore and an economically important invasive pest in North America and Europe. In visual surveys of 220 plant taxa in commercial nurseries in Maryland, USA, H. halys was more abundant on non-Asian plants and selected these over Asian plants. The relationship between the relative use of plants and their availability was strongly positive but depended also on plant origin at two of our three sites, where the higher relative use of non-Asian plants was greatest for highly abundant taxa. These results highlight the importance of considering both plant origin and relative abundance in understanding the selection of host plants by invasive generalist herbivores in diverse, natural and urban forests.
With increased global trade, exotic species have become widespread and continue to pose serious threats to biodiversity worldwide1,2. Herbivorous insects are among the most economically and ecologically damaging exotic species, leading to billions of $US in losses and management costs annually3,4. As exotic plants also make up a substantial proportion of the species in most terrestrial ecosystems5, understanding and predicting how exotic herbivores will interact with native and exotic plant species in their invaded ranges is becoming increasingly important.
Several ecological and evolutionary arguments explicate how native and exotic plants and herbivores are likely to interact based on their geographic origins6,7,8. Most herbivorous insects are specialists, feeding on plants within a single family9. Because these herbivores may not have adaptations to feed on novel plants, host shifts may be limited10. Thus, exotic herbivores encountering a suite of potential host plants may select plants from their original range or close relatives of those plants in the newly invaded range.
On the other hand, plants with little or no shared co-evolutionary history with an herbivore may be more susceptible to herbivory from a novel consumer. The concept of evolutionary naiveté has received significant attention in the invasion literature and is integral to the formulation of hypotheses such as “biotic resistance”11, “new associations”12, and “defense free space”8. These hypotheses predict that an exotic consumer will preferentially use naïve resources in their invaded range, due to a lack of evolved defenses.
Recent work has demonstrated that the geographic origins of plants and herbivores are frequently important in determining resource consumption and ecological impacts in the invaded range, but consumer diet breadth and phylogenetic relationships among plants modify these expectations13,14. Specifically, monophagous and oligophagous consumers generally suffer fitness losses on novel host plants, especially when novel hosts are phylogenetically distant from their ancestral hosts14. Polyphagous consumers, on the other hand, often experience fitness gains on novel plants, regardless of phylogenetic relatedness among ancestral and novel hosts14.
Exotic generalist herbivores establish successfully, enjoy fitness gains in the invaded range, and have broad ecological impacts on diverse host plant taxa15,16. Understanding and predicting the use of host plants by exotic generalist herbivores tackles a formidable ecological and management problem. As a null hypothesis, a polyphagous consumer may feed according to the availability of resources in the landscape17. Deviations from this null expectation can indicate electivity, or selection for or against certain plant taxa given the available resources18. How resource availability and origin influence host plant use by introduced generalist consumers remains untested. Such knowledge is essential for predicting and mitigating the impacts of broadly feeding, introduced consumers.
Here, we test whether plant origin and availability singly or interactively influence the abundance and electivity of the herbivorous stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), across diverse trees and shrubs in commercial plant nurseries. Originally native to eastern Asia, H. halys is highly invasive and broadly polyphagous, feeding on and damaging field and vegetable crops, tree fruits, and ornamental and wild plants19,20. Using three years of repeated visual surveys of H. halys, we demonstrate that plant origin, while important, needs to be considered along with plant relative abundance to understand how invasive generalist consumers use novel resources.
Results
Effect of plant origin
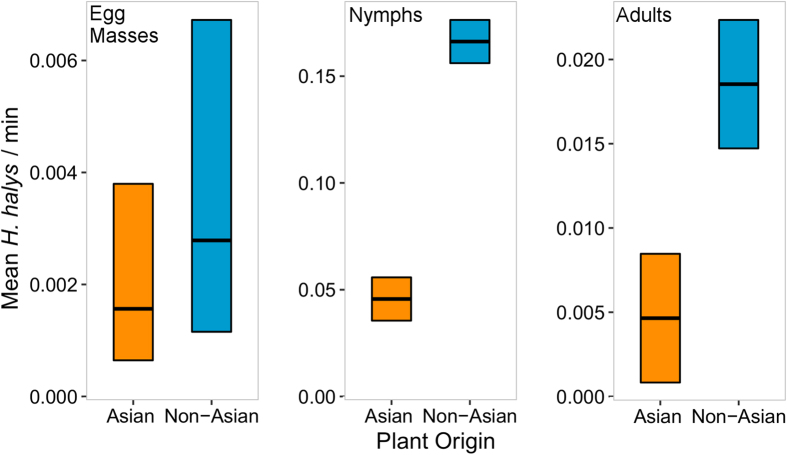
The abundance of each life stage of H. halys was significantly influenced by plant origin (Fig. 1). Mean abundance per survey was significantly higher on plant taxa of non-Asian compared with those of Asian origin for egg masses (Wald’s χ2 = 8.95, df = 1, P = 0.0028), nymphs (F test; values in parentheses denote total observations: F(594) = 26.87, P < 0.0001), and adults (F(594) = 10.91, P = 0.0010; Fig. 1).
Figure 1. Mean abundances (number per 1 min. survey) of egg masses, nymphs, and adults of Halyomorpha halys for plants of non-Asian (blue) and Asian (orange) origins.
The mean estimates were derived from Poisson-lognormal GLMM (egg masses) and LMEs (nymphs and adults). Plotted are back transformed model estimated means (thickened horizontal line) and SE (log link function for egg mass, and cube root transformation for nymphs and adults). Tukey’s post hoc comparisons are all significant at α = 0.05.
Relative use of plants
The relative use of plant taxa by H. halys at our study sites depended on the combination of plant relative abundance and origin (Fig. 2). At Raemelton Farm, our most diverse site, the relative use of plants was an increasing function of plant relative abundance and was higher for non-Asian compared to Asian plants (Fig. 2). The most parsimonious model included the additive fixed effects of plant origin (F(496) = 11.09, P = 0.0009) and plant relative abundance (F(496) = 537.97, P < 0.0001). At Ruppert East, the relationship between relative use and relative abundance depended on plant origin (significant interactive effect: F(40) = 9.87, P = 0.0007; Fig. 2). Specifically, the slope of the relationship between relative use and relative abundance was steeper for plants with non-Asian (b1 = 1.43) compared to Asian (b1 = 0.55) origins. Similarly, a significant interaction between relative abundance and plant origin was evident at Ruppert North, such that the higher relative use of non-Asian plants became more pronounced with increasing plant relative abundance (significant interactive effect: F(58) = 6.468, P = 0.0145; Fig. 2). Again, the slope of the relationship between relative use and relative abundance for non-Asian plants (b1 = 1.05) was steeper than that for Asian plants (b1 = 0.51). Because the ranges of p differed for Asian and non-Asian plants at this site, we examined a reduced data set of taxa for which p < 0.06 (the maximum for Asian plants). The interactive effect remained significant for this subset of the data, with an increasing difference in relative use between Asian and non-Asian taxa with increasing relative abundance (F(51) = 10.75, P = 0.0022).
Figure 2. Relationships between relative abundance, relative use, and plant origin by site.
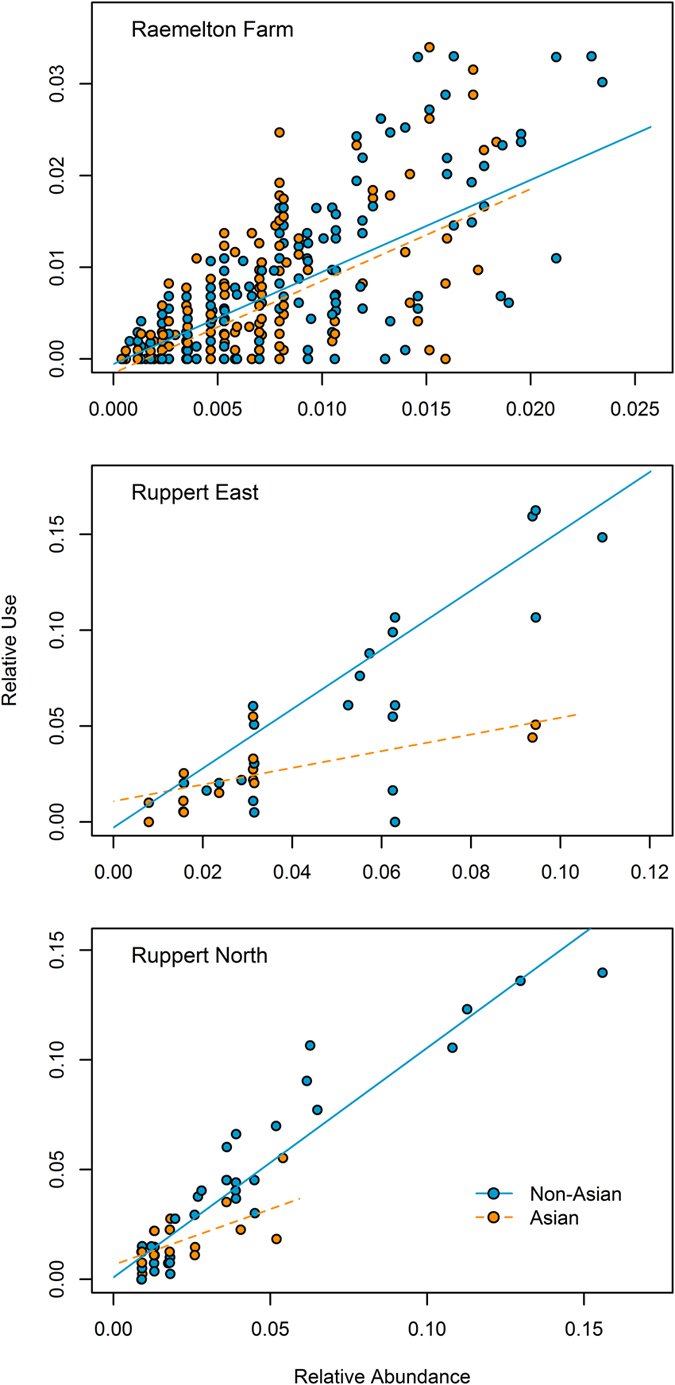
Lines show relationships estimated from mixed models (incorporating random effects of year and taxonomic factors; see Methods for exact model specifications), and points show relative use for each plant taxon each year of sampling for non-Asian (blue symbols, solid lines) and Asian (orange symbols, dashed lines) taxa.
Electivity
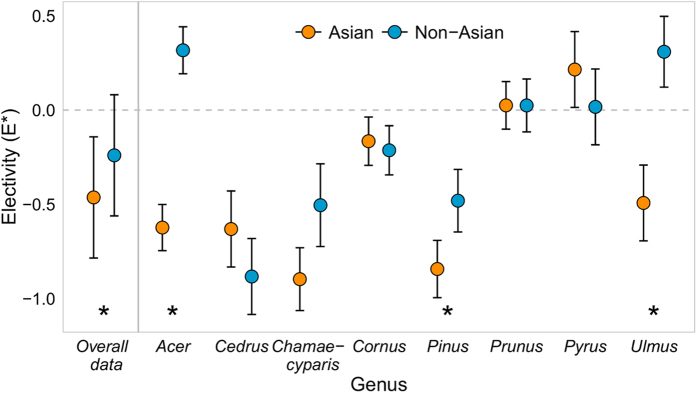
Electivity was higher among taxa with non-Asian compared to Asian origins for the full data set (F(594) = 31.48, P < 0.0001; Fig. 3, Overall data). For the genus-level analysis, the effect of plant origin on electivity depended strongly on plant genus (significant interactive effect: F(212) = 9.77, P < 0.0001; Fig. 3). Tukey’s post hoc comparisons revealed significantly higher electivity for non-Asian than Asian taxa in the genera Acer, Pinus, and Ulmus (adjusted P < 0.05) and slightly higher electivity for non-Asian taxa in the genus Chamaecyparis (adjusted P = 0.097). In particular, non-Asian Acer and Ulmus were strongly selected by H. halys (positive values of E*) in these landscapes.
Figure 3. Electivity (E*) across the full data set (Overall data) and for the subset of genera with at least 2 taxa with Asian and 2 taxa with non-Asian origins (genus-level analysis).
Plotted are model estimated means and SE; significance of Tukey’s post hoc comparisons within genera (P < 0.05) are indicated with an *.
Discussion
Accidental and purposeful introductions of both plants and herbivores have set the stage for a myriad of potential new consumer-resource interactions. Predicting interactions in such non-coevolved systems is a major challenge in modern ecology21. Our results identify the roles of both plant origin and abundance on use and electivity by the exotic generalist H. halys. Exotic consumers and pathogens are frequently able to exploit resources in their invaded ranges, often due to a lack of co-evolved defenses8. Prime examples of this phenomenon, however, have typically involved relatively specialized consumers: the introduced Eurasian viburnum leaf beetle, Pyrrhalta viburni (Paykull) exploits North American Viburnum spp L.22; the Asian emerald ash borer, Agrilus planipennis Fairmaire, is currently decimating North American ash trees (Fraxinus spp L.)23; and the introduced chestnut blight, Cryphonectria parasitica (Murrill) Barr, has eliminated American chestnut, Castanea dentata (Marshall) Borkh, from eastern forests24.
Whereas introduced specialist consumers may feed on resources which are phylogenetically or chemically similar to those found in their native ranges13,14, the diet of introduced generalist consumers is more difficult to predict. Due to their ability to tolerate a broad range of host defenses or suppress plant defenses, polyphagous herbivores may be even better able to make use of novel plants than specialists25,26. Thus, when polyphagous herbivores are introduced, they tend to incur fewer negative fitness consequences from feeding on naïve hosts14 and may generally prefer native plants27. In our three-year field study, the highly polyphagous H. halys was more abundant on plants not present in its native range (Fig. 1) and selected these over Asian plants (Fig. 2). Similarly, Morrison and Hay28 demonstrated that generalist apple snails and crayfish preferentially selected aquatic plants with which they shared little co-evolutionary history. Thus, in habitats with both exotic and native plants, it is clear that host shifting by introduced generalist herbivores is common and can be detrimental to the health of native plants. In the case of H. halys, which specializes in attacking reproductive organs of plants29, preference for plants native to the invaded realm portends broader ecological consequences such as invasional meltdown.
In addition to the geographic origin of plants, plant availability strongly influenced the relative use of plants by H. halys in this study and was a significant factor across all sites (Fig. 2). A null expectation for a generalist consumer encountering multiple potential resources would be a one-to-one relationship between relative use and relative abundance17, and this is the case for some polyphagous herbivores. Working with the polyphagous fall webworm, Hyphantria cunea (Drury), Mason et al.30 documented that host plant use in eastern forests was governed largely by the relative abundance of plants. Polyphagous species often, however, exhibit electivity, selecting or avoiding particular host plants and deviating from expectations based solely on plant availability31. In our study, variation around the expected one-to-one line (i.e., electivity) was evident across all sites and in the data set as a whole, but the specific relationship between relative use and relative abundance differed among sites. Notably, the effects of plant origin and relative abundance were additive at our highly diverse site (Fig. 2, Raemelton Farm) but interactive at two less-diverse sites (Fig. 2, Ruppert East, Ruppert North; Table 1). Relative use of any given taxon by H. halys at the highly diverse Raemelton site was on average quite low (note the difference in axis scales among sites in Fig. 2), with extensive variation around the regression lines. We attribute such low values of relative use to the very high plant diversity at Raemelton, with each taxon contributing only a small portion of the total plant abundance at the site.
Table 1. Summary of sampling across plant genera and taxa (species and cultivated varieties) and the mean (min, max) relative abundance (p), relative use (r), and electivity (E*) of plants in Maryland nurseries by Halyomorpha halys for each site and year.
| Site | Year | Genera | Taxa | p | r | E* |
|---|---|---|---|---|---|---|
| Raemelton Farm | 2011 | 54 | 174 | 0.006 (0.001, 0.02) | 0.006 (0, 0.025) | −0.104 (−1, 0.241) |
| 2012 | 52 | 172 | 0.006 (0, 0.023) | 0.006 (0, 0.043) | −0.283 (−1, 0.566) | |
| 2013 | 55 | 196 | 0.005 (0, 0.023) | 0.005 (0, 0.034) | −0.239 (−1, 0.469) | |
| Ruppert East | 2012 | 15 | 23 | 0.043 (0.016, 0.109) | 0.043 (0.005, 0.159) | −0.081 (−0.835, 0.347) |
| 2013 | 17 | 25 | 0.04 (0.008, 0.094) | 0.04 (0, 0.162) | −0.093 (−1, 0.293) | |
| Ruppert North | 2012 | 16 | 26 | 0.038 (0.013, 0.156) | 0.038 (0.004, 0.14) | −0.054 (−0.557, 0.261) |
| 2013 | 20 | 39 | 0.026 (0.009, 0.113) | 0.026 (0, 0.123) | −0.076 (−1, 0.27) |
Relative use and electivity were calculated based on the presence of any life stage of H. halys on trees.
The importance of considering the joint effects of both plant abundance and plant origin is evident at the other two sites. There, plant relative use increased more steeply with plant abundance for non-Asian compared to Asian plants (Fig. 2), leading to large differences in use between abundant non-Asian and abundant Asian plants. We also found evidence that H. halys selects non-Asian taxa in the genera Acer L. and Ulmus L. (Fig. 3), trees which can be exploited for sugar rich exudates even when reproductive structures are not present32. Together, our results suggest that the risk posed by H. halys to trees may be exacerbated for abundant maples (Acer spp), elms (Ulmus spp), and pines (Pinus spp L.) in eastern forests (Fig. 3) particularly in low diversity environments such as the urban forest. This finding, which bears investigation at additional sites and in other habitats invaded by H. halys, may indicate increased risk for dominant native trees. Increasing the diversity of tree species and cultivars in managed environments such as urban forests and landscapes may be increasingly important in buffering against losses to introduced insect pests33. Furthermore, our results highlight the importance of jointly considering the geographic origin and relative abundance of potential resources when assessing novel consumer-resource interactions in invaded habitats.
Methods
Field surveys
We conducted repeated one-minute visual surveys of trees and shrubs in commercial nurseries in Maryland, USA, from late May through early August, 2011–2013. In each survey, we recorded the number of egg masses, nymphs, and adults of H. halys on the leaves, fruits and flowers, and bark of the tree up to a height of 3 m. In 2011, we conducted four surveys per tree at Raemelton Farm in Frederick County, MD (39.299468°N, 77.458549°W). In 2012 and 2013, we conducted six surveys per tree and included two additional sites at Ruppert Nurseries in Montgomery County, MD (Ruppert East: 39.238821°N, 77.138334°W; Ruppert North: 39.251091°N, 77.157074°W). At each site, trees of a particular species or cultivated variety (hereafter ‘taxa’) are planted within a row of 25–35 trees, but different rows within a field are typically planted with different taxa. For 3–4 fields per site each year, we conducted spatially stratified surveys on three edge trees (0–5 m from the field edge) and three interior (15–20 m from the edge) trees in each row to mitigate the influence of known edge effects34,35,36.
Definitions and data set construction
We calculated the abundance, relative use, and electivity of H. halys separately for each plant taxon. Because the available plants varied across sites and over time as trees were sold and replanted, all calculations were made for taxa from each site and year separately. Thus, the experimental unit throughout the analysis is a unique plant taxon at a site in a year. To calculate the abundance of H. halys for each taxon, we summed the abundance of each life stage (egg masses, nymphs, and adults separately) across all surveys conducted on each plant taxon within a year for each site. These summed abundances were used used in conjunction with the total number of surveys conducted for each taxon to calulate the mean number of individuals (or egg masses) per 1-min. survey.
We calculated the relative abundance (p) of each plant taxon as the total number of individuals of the taxon divided by the total number of individual trees of all taxa surveyed at a site for a given year. This measure of plant availability assumes that the number of trees surveyed is an unbiased sample of all the trees at the site, a reasonable assumption given that we equally surveyed all rows of a field, and that the sampled fields were representative of the plant diversity and composition at each site. We calculated the relative use (r) of each taxon as the number of trees of the taxon that were used by H. halys, divided by the total number of trees used by H. halys at a site in a year. Use of a taxon was defined as the presence of any life stage on a tree of that taxon during any of the sampling periods within a year. As with abundance, both p and r were calculated within each site each year.
Electivity measures the relative use of resources after accounting for their relative abundances17,18. We calculated indices of electivity for each plant taxon i at each site in each year using r and p. We used Vanderploeg and Scavia’s relativized electivity index, 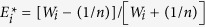 , where
, where 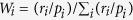 and n is the number of plant taxa available37. E* was the most suitable electivity index for our purposes because: 1) it best met model assumptions for our analysis; 2) it is less sensitive than the forage ratio, FRi = ri/pi, and Ivelev’s electivity, Ei =(ri − pi)/(ri + pi)18, to values of n; and 3) it has a straight-forward interpretation, ranging in value from negative one for resources which are completely avoided, to positive one for resources which are used exclusively. All values of E*, E, and FR were highly correlated in our data set (Pearson’s ρ > 0.93, P < 0.0001 for pairwise correlations; see also Lechowicz37).
and n is the number of plant taxa available37. E* was the most suitable electivity index for our purposes because: 1) it best met model assumptions for our analysis; 2) it is less sensitive than the forage ratio, FRi = ri/pi, and Ivelev’s electivity, Ei =(ri − pi)/(ri + pi)18, to values of n; and 3) it has a straight-forward interpretation, ranging in value from negative one for resources which are completely avoided, to positive one for resources which are used exclusively. All values of E*, E, and FR were highly correlated in our data set (Pearson’s ρ > 0.93, P < 0.0001 for pairwise correlations; see also Lechowicz37).
Plant origin (following Dirr38) was assigned as follows: taxa originally from the native range of H. halys (i.e., China, Taiwan, Japan, Korea19) were considered “Asian”; taxa not originally from that range were considered “non-Asian.” Taxa with hybrid Asian and non-Asian origins or with unknown origins (i.e., open-pollinated Malus taxa) were used in calculations of r, p, and E* but were dropped from the analysis of plant origin. In total, we conducted 47,136 one-minute surveys on 4,620 unique trees. Excluding species of hybrid or unknown origins yielded a final data set of 594 observations of the mean abundance, relative use, and electivity of H. halys on 220 unique taxa from 58 genera of plants. Please see Table 1 for a summary of the sampling effort at each site and Supplementary Table S1 for the full data table.
Statistical analysis
All statistical analyses were conducted in the program R39. To assess the effects of plant origin on the abundance of H. halys, we constructed separate mixed effects models for egg masses, nymphs, and adults using the lme4 package40. Model building and selection procedures for the mixed effects modeling followed the approach used by Zuur et al.41. For each model, we considered a set of random effects that included the following terms: plant classification (angiosperm or gymnosperm), plant genus, genus within classification category, plant taxon, and site-year combination. We then selected the best set of random effects as the model with the lowest AIC (Akaike’s Information Criterion) value prior to the assessment of fixed effects.
For the analysis of egg mass abundance, we constructed a generalized linear mixed model (GLMM) based on Laplace approximation, with a Poisson-lognormal error distribution and log link function42,43. This GLMM included the following terms: total egg mass abundance per plant taxon at a site in a year as the response variable, an offset for the number of surveys conducted for each plant taxon, and the fixed effect of plant origin. The final set of random effects for this model included the site-year combination to account for repeated measures and an observation-level variable to account for overdispersion (see supplementary material in Bolker et al.43). We found no evidence for additional oversdispersion or correlations among random effect terms in the final model, and visual plots of the variances in a location-scale plot with superimposed loess fit demonstrated that the assumptions of the model were met appropriately. The statistical significance of this egg mass GLMM model was determined with a Wald’s chi-square test; significance for all tests was evaluated using an α-level threshold of 0.05.
For the abundance of nymphs and adults, we constructed separate linear mixed effects models (LME’s). The response variable of mean abundance per one-minute survey was cube-root transformed to normalize residuals for the nymph and adult abundance analyses. The appropriateness of the final models was confirmed using diagnostic plots visualizing within-group residuals (standardized residuals vs. fitted values, normal Q-Q plots, and histograms of residuals) and estimated random effects (normal Q-Q plots and pairs-scatterplot matrix)40. Final random effects for both nymph and adult models included genus within classification and site-year combination. We used F tests to assess the significance of fixed effects and compared model estimated means through Tukey’s post hoc tests.
We next modeled relative use as a function of the fixed effects of plant relative abundance, plant origin, and their interaction. Sites were analyzed separately due to widely differing plant diversity and ranges of relative abundance and relative use (see Table 1); for Raemelton Farm, the best model fit was achieved by square root transforming values for relative use and relative abundance. For each site, we again selected the most parsimonious set of random effects via comparison of AIC values and then evaluated the significance of fixed effects with F tests. The best models for both Raemelton Farm and Ruppert East included random effects terms for genus within classification and year; the model for Ruppert North included the random effect of plant taxon.
For electivity, we first performed a global analysis of the full data set, comparing E* of Asian and non-Asian plants. Random effects for the final model included site-year combination and genus within classification. Because previous investigations found substantial differences in the mean abundance of H. halys among cultivars and species36,44, we next performed a genus-level analysis, asking whether differences in electivity between Asian and non-Asian plants were consistent across genera. For this analysis, we used the subset of genera with at least two taxa of Asian origin and two of non-Asian origin; all such genera were represented only at Raemelton Farm. We rescaled r and p to sum to 1 for each year for this subset of taxa only, and recalculated E*. The values of E* for this subset were highly correlated with those from the full data set (Pearson’s ρ = 0.9995, P < 0.0001) but allow a clearer test of selection for and against these particular taxa. Final random effects for this model included terms for year and plant classification.
Additional Information
How to cite this article: Martinson, H. M. et al. Invasive stink bug favors naïve plants: Testing the role of plant geographic origin in diverse, managed environments. Sci. Rep. 6, 32646; doi: 10.1038/srep32646 (2016).
Supplementary Material
Acknowledgments
We are grateful to R. Wallace, C. Brodo, D. Reisinger, S. Harris, K. Beiter, C. McMullen, B. Cornwell, and K. Keochinda for field assistance, C. Sargent for logistical support, and S. Black, K. Lewis, and N. Graves for field access. This work was funded by grants from the USDA National Institute of Food and Agriculture (SCRI Award 2011-51181-30937, McIntire Stennis Grant MD-ENTM-04168757).
Footnotes
Author Contributions All authors contributed to the design of the study. H.M.M., E.J.B., C.B.R. and M.J.R. collected the field data through surveys. H.M.M. and P.D.V. analyzed the data. All authors contributed to discussions and to the writing of the article.
References
- Mack R. N. et al. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 10, 689–710 (2000). [Google Scholar]
- Liebhold A. & Wingfield M. Globalization and its implications to forest health. For. Glob. Chall. Oppor. Sustain. Dev. 36 (2014). [Google Scholar]
- Holmes T. P., Aukema J. E., Von Holle B., Liebhold A. & Sills E. Economic impacts of invasive species in forests: past, present, and future. Ann. N. Y. Acad. Sci. 1162, 18–38 (2009). [DOI] [PubMed] [Google Scholar]
- Aukema J. E. et al. Economic impacts of non-native forest insects in the continental United States. PLOS ONE 6, e24587 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asner G. P. et al. Invasive plants transform the three-dimensional structure of rain forests. Proc. Natl. Acad. Sci. 105, 4519–4523 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron J. L. & Vilà M. When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95, 361–373 (2001). [Google Scholar]
- Keane R. M. & Crawley M. J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 17, 164–170 (2002). [Google Scholar]
- Gandhi K. J. K. & Herms D. A. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol. Invasions 12, 389–405 (2010). [Google Scholar]
- Forister M. L. et al. The global distribution of diet breadth in insect herbivores. Proc. Natl. Acad. Sci. 112, 442–447 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallamy D. W. Do alien plants reduce insect biomass? Conserv. Biol. 18, 1689–1692 (2004). [Google Scholar]
- Parker J. D. & Hay M. E. Biotic resistance to plant invasions? Native herbivores prefer non-native plants. Ecol. Lett. 8, 959–967 (2005). [DOI] [PubMed] [Google Scholar]
- Hokkanen H. M. & Pimentel D. New associations in biological control: theory and practice. Can. Entomol. 121, 829–840 (1989). [Google Scholar]
- Pearse I. S. & Hipp A. L. Phylogenetic and trait similarity to a native species predict herbivory on non-native oaks. Proc. Natl. Acad. Sci. 106, 18097–18102 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertheau C., Brockerhoff E. G., Roux-Morabito G., Lieutier F. & Jactel H. Novel insect-tree associations resulting from accidental and intentional biological ‘invasions’: a meta-analysis of effects on insect fitness. Ecol. Lett. 13, 506–515 (2010). [DOI] [PubMed] [Google Scholar]
- Liebhold A. M., MacDonald W. L., Bergdahl D. & Mastro V. C. Invasion by exotic forest pests: a threat to forest ecosystems. For. Sci. 41, a0001–z0001 (1995). [Google Scholar]
- McKinney M. L. & Lockwood J. L. Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 14, 450–453 (1999). [DOI] [PubMed] [Google Scholar]
- Singer. Reducing ambiguity in describing plant–insect interactions: ‘preference’, ‘acceptability’ and ‘electivity’. Ecol. Lett. 3, 159–162 (2000). [Google Scholar]
- Ivlev V. S. Experimental ecology of the feeding of fishes. (Yale University Press, 1961). [Google Scholar]
- Hoebeke E. R. & Carter M. E. Halyomorpha halys (Stål) (Heteroptera: Pentatomidae): a polyphagous plant pest from Asia newly detected in North America. Proc. Entomol. Soc. Wash. 105, 225–237 (2003). [Google Scholar]
- Rice K. B. et al. Biology, ecology, and management of brown marmorated stink bug (Hemiptera: Pentatomidae). J. Integr. Pest Manag. 5, A1–A13 (2014). [Google Scholar]
- Pearse I. S., Harris D. J., Karban R. & Sih A. Predicting novel herbivore–plant interactions. Oikos 122, 1554–1564 (2013). [Google Scholar]
- Desurmont G. A., Donoghue M. J., Clement W. L. & Agrawal A. A. Evolutionary history predicts plant defense against an invasive pest. Proc. Natl. Acad. Sci. 108, 7070–7074 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms D. A. & McCullough D. G. Emerald ash borer invasion of North America: history, biology, ecology, impacts, and management. Annu. Rev. Entomol. 59, 13–30 (2014). [DOI] [PubMed] [Google Scholar]
- Anagnostakis S. L. The effect of multiple importations of pests and pathogens on a native tree. Biol. Invasions 3, 245–254 (2001). [Google Scholar]
- Cornell H. V. & Hawkins B. A. Herbivore responses to plant secondary compounds: a test of phytochemical coevolution theory. Am. Nat. 161, 507–522 (2003). [DOI] [PubMed] [Google Scholar]
- Ali J. G. & Agrawal A. A. Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci. 17, 293–302 (2012). [DOI] [PubMed] [Google Scholar]
- Parker J. D., Burkepile D. E. & Hay M. E. Opposing effects of native and exotic herbivores on plant invasions. Science 311, 1459–1461 (2006). [DOI] [PubMed] [Google Scholar]
- Morrison W. E. & Hay M. E. Herbivore preference for native vs. exotic plants: generalist herbivores from multiple continents prefer exotic plants that are evolutionarily naïve. PLOS ONE 6, e17227 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinson H. M., Venugopal P. D., Bergmann E. J., Shrewsbury P. M. & Raupp M. J. Fruit availability influences the seasonal abundance of invasive stink bugs in ornamental tree nurseries. J. Pest Sci. 88, 461–468 (2015). [Google Scholar]
- Mason P. A., Wilkes S. R., Lill J. T. & Singer M. S. Abundance trumps quality: bi-trophic performance and parasitism risk fail to explain host use in the fall webworm. Oikos 120, 1509–1518 (2011). [Google Scholar]
- Singer M. & Stireman J. How foraging tactics determine host-plant use by a polyphagous caterpillar. Oecologia 129, 98–105 (2001). [DOI] [PubMed] [Google Scholar]
- Martinson H. M., Raupp M. J. & Shrewsbury P. M. Invasive stink bug wounds trees, liberates sugars, and facilitates native Hymenoptera. Ann. Entomol. Soc. Am. 106, 47–52 (2013). [Google Scholar]
- Raupp M. J., Cumming A. B. & Raupp E. C. Street tree diversity in eastern North America and its potential for tree loss to exotic borers. Arboric. Urban For. 32, 297 (2006). [Google Scholar]
- Venugopal P. D., Coffey P. L., Dively G. P. & Lamp W. O. Adjacent habitat influence on stink bug (Hemiptera: Pentatomidae) densities and the associated damage at field corn and soybean edges. PLOS ONE 9, e109917 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal P. D., Martinson H. M., Bergmann E. J., Shrewsbury P. M. & Raupp M. J. Edge effects influence the abundance of the invasive Halyomorpha halys (Hemiptera: Pentatomidae) in woody plant nurseries. Environ. Entomol. 44, 474–479 (2015). [DOI] [PubMed] [Google Scholar]
- Bergmann E. J., Venugopal P. D., Martinson H. M., Raupp M. J. & Shrewsbury P. M. Host plant use by the invasive Halyomorpha halys (Stål) on woody ornamental trees and shrubs. PLOS ONE 11, e0149975 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechowicz M. J. The sampling characteristics of electivity indices. Oecologia 52, 22–30 (1982). [DOI] [PubMed] [Google Scholar]
- Dirr M. A. Manual of woody landscape plants. (Stiples, 2009). [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. (The R foundation for statistical computing, 2015). [Google Scholar]
- Bates D., Mächler M., Bolker B. & Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- Zuur A. F., Ieno E. N., Walker N., Saveliev A. A. & Smith G. M. Mixed effects models and extensions in ecology with R. (Springer, 2009). [Google Scholar]
- Elston D. A., Moss R., Boulinier T., Arrowsmith C. & Lambin X. Analysis of aggregation, a worked example: numbers of ticks on red grouse chicks. Parasitology 122, 563–569 (2001). [DOI] [PubMed] [Google Scholar]
- Bolker B. M. et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 (2009). [DOI] [PubMed] [Google Scholar]
- Blaauw B. R., Polk D. & Nielsen A. L. IPM-CPR for peaches: incorporating behaviorally-based methods to manage Halyomorpha halys and key pests in peach. Pest Manag. Sci. 71, 1513–1522 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.