Fig. 1.
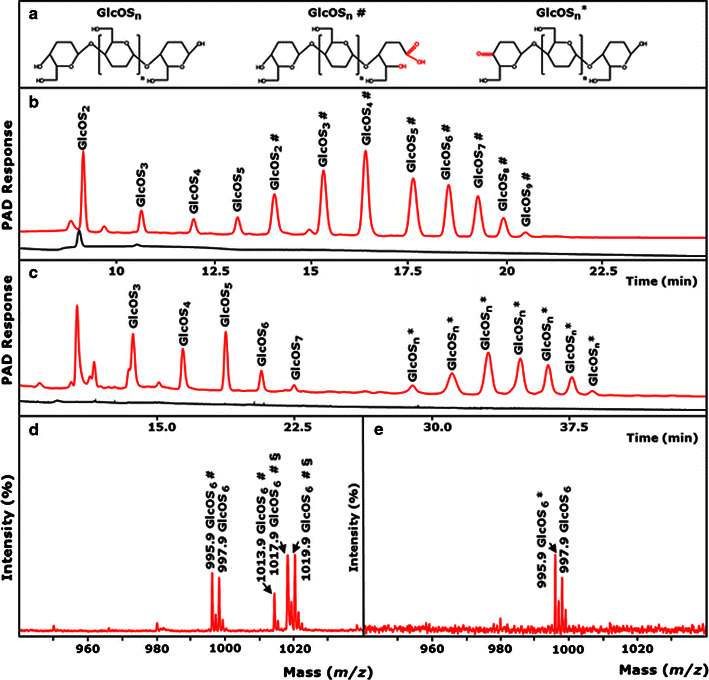
Activity of MtLPMO9B and MtLPMO9C on regenerated amorphous cellulose. a Structure and nomenclature of released non-oxidized and C1- and C4-oxidized gluco-oligosaccharides (GlcOSn, GlcOS#n, GlcOS*n, respectively). HPAEC elution pattern of regenerated amorphous cellulose (RAC; 2 mg × mL−1) incubated with b MtLPMO9B (10 mg × g−1 substrate) and c MtLPMO9C (10 mg × g−1 substrate), in the presence (1 mM, red line) and absence of ascorbic acid (black line). A different gradient was used for the separation of b C1- and c C4-oxidized gluco-oligosaccharides (See “Methods”). The C4-oxidized gluco-oligosaccharides are known to be unstable under the alkaline conditions present during HPAEC analysis and undergo further derivatization to gem-diols, which are actually annotated as C4-oxidized gluco-oligosaccharides (GlcOS*n) [34]. MALDI-TOF mass spectrum (m/z values) of RAC incubated with d MtLPMO9B or e MtLPMO9C, in the presence of ascorbic acid. d Double Li− adducts of C1-oxidized gluco-oligosaccharides are marked with section symbol. d Oxidation of the C1-carbon atom results in the formation non-oxidized gluco-oligosaccharides (GlcOSn) and C1-oxidized gluco-oligosaccharides present as a δ-lactone (−2 Da, marked as GlcOS#n). Lactones are unstable and convert to aldonic acids by the addition of water, leading to a 16 Da higher mass compared to the non-oxidized gluco-oligosaccharide (+16 Da, marked as GlcOS#n). Double Li− adducts of C1-oxidized gluco-oligosaccharides are marked with section symbol (GlcOS#6§, 1019.9 and 1017.9 Da, probably due to double oxidation). e Oxidation of the C4-carbon atom results in the formation of non-oxidized gluco-oligosaccharides (GlcOSn) and C4-oxidized gluco-oligosaccharides present as ketoaldoses (−2 Da, marked as GlcOS*n). No gem-diols were formed. For more information see “Methods”
