Abstract
Monocytes are mediators of the inflammatory response and include three subsets: classical, intermediate, and nonclassical. Little is known about the phenotypical and functional age-related changes in monocytes and their association with soluble inflammatory biomarkers, cytomegalovirus infection, and functional and mental decline. We assayed the activation ex vivo and the responsiveness to TLR2 and TLR4 agonists in vitro in the three subsets and assessed the intracellular production of IL1-alpha (α), IL1-beta (β), IL-6, IL-8, TNF-α, and IL-10 of elderly adults (median 83 [67–90] years old; n = 20) compared with young controls (median 35 [27–40] years old; n = 20). Ex vivo, the elderly adults showed a higher percentage of classical monocytes that expressed intracellular IL1-α (p = .001), IL1-β (p = .001), IL-6 (p = .002), and IL-8 (p = .007). Similar results were obtained both for the intermediate and nonclassical subsets and in vitro. Polyfunctionality was higher in the elderly adults. The functionality ex vivo was strongly associated with soluble inflammatory markers. The activation phenotype was independently associated with the anti-cytomegalovirus IgG levels and with functional and cognitive decline. These data demonstrate that monocytes are key cell candidates for the source of the high soluble inflammatory levels. Our findings suggest that cytomegalovirus infection might be a driving force in the activation of monocytes and is associated with the functional and cognitive decline.
Key Words: Inflammation, Aging, Monocyte function, CMV, Cognitive, Mini-mental
Aging is associated with the chronic increase in systemic inflammatory status (inflammaging) (1). Inflammatory disorders are a major cause of age-related diseases such as Alzheimer’s and cardiovascular diseases, which are important causes of increased mortality in elderly adults (2,3). These illnesses have been associated with the chronic increase in soluble inflammatory and coagulation markers, T-cell phenotype alterations, and cytomegalovirus (CMV) infection (4–6). Recent studies have focused on developing an improved understanding of the changes in innate immune cells revealing profound deregulations in the blood mononuclear phagocyte system with aging (7). Human monocytes are innate immune cells that are characterized by a high degree of heterogeneity and complexity. Monocytes include three subsets: the classical (CD14++CD16−), the intermediate (CD14++CD16+), and the nonclassical (CD14dimCD16+); they differ in size, morphology, phenotype, and function. The classical and intermediate subsets secrete high levels of proinflammatory cytokines in response to microbial products, and the nonclassical subset appears to “patrol” the vessel walls (8). However, data on the phenotype and functional age-related changes in monocytes and the association of these parameters with soluble inflammatory and coagulation biomarkers are scarce.
CMV is extremely prevalent in older people, and this virus has been identified in areas of the brain in individuals affected by Alzheimer’s disease as a likely cause of this type of dementia (9). Even when CMV is in a latent state, inflammatory processes are ongoing, and viral reactivation leads to cytokine accumulation, resulting in direct damage to neurons (10). In this regard, latent CMV has been shown to be highly immunostimulatory in monocytes and macrophages (11,12), which results in heightened levels of circulating inflammatory cytokines. Recently, it has been described that monocyte activation and recruitment is associated with Alzheimer’s amyloid pathology in mouse models (13). Nonetheless, basic functionality studies of human monocytes in elderly adults and the functional relationship to CMV infection, which eventually leads to functional and cognitive impairment in elderly adults, are unknown.
Thus, the aim of the present study was to analyze the phenotype and functional changes in the three monocyte subsets with aging and to study the relationship of these changes with the high persistent soluble inflammatory state, latent CMV infection, and functional and neurocognitive decline in elderly adults.
Method
Study Participants
Consecutive nursing home residents from Seville (Spain), between May 26, 2014 and July 3, 2014, were asked to participate in the HELIOPOLIS cohort. The inclusion criteria were being aged 65 years or older and having a self-sufficient health status. The exclusion criteria included any of the following situations during the preceding 6 months: (i) clinical data indicating active infections, (ii) hospital admission, (iii) anti-tumor therapy, or (iv) any treatment that could influence the immune status (mainly corticosteroids). The elderly group (Elderly, n = 20) was compared with the young healthy volunteers, who made up the control group (Young, n = 20). Laboratory evaluations were performed at the Laboratory of Immunovirology, Institute of Biomedicine, Virgen del Rocío University Hospital in Seville (Spain). All necessary institutional or ethical review board approvals were obtained, and written informed consent was obtained from all study participants.
Lymphocyte Count
The absolute CD4+, CD8+ T-cell counts (cells/mm3) and the CD4:CD8 ratio were determined using an Epics XL-MCL flow cytometer (Beckman-Coulter, Brea, CA) according to the manufacturer’s instructions.
Immunophenotyping and Intracellular Cytokine Staining of Monocytes
One milliliter samples of peripheral fresh whole blood samples were collected in ethylene diamine tetra-acetic acid tubes (within 30min prior to the assay). Erythrocytes were lysed according to the manufacturer’s instructions (Lyse Buffer, R&D, San Diego, CA), and the cells were immediately immunophenotyped using a panel of antibodies for lineage, activation, cell adhesion surface markers, and viability dye to exclude nonviable cells: LIVE/DEAD fixable Violet Dead Cell Stain, CD8-Qdot605, CD14-Qdot655, CD19-PB, and CD40-APC (Life Technologies, Carlsbad, CA); CD3-APC-H7, CD4-BV786, CD56-PB, CD16-PE-CF595, CD11b-Alexa700, CD62L-PE, and CD49d-FITC (BD Biosciences, Franklin Lakes, NJ); and HLA-DR-BV570, CD38-PerCPCy5.5 and CD163-PCy7 (Biolegend, San Diego, CA). Isotype controls for CD14 (Life Technologies), CD16, CD11b, CD62L, and CD49d (BD Biosciences) and CD38 and CD163 (Biolegend) were included in each experiment.
Intracellular cytokine staining was performed on 1.5ml of whole blood that was collected in ethylene diamine tetra-acetic acid tubes. Erythrocytes were lysed according to the manufacturer’s instructions (Lyse Buffer); the cells were washed twice with the washing buffer provided by the kit and were then washed with phosphate-buffered saline without calcium and magnesium and a maximum endotoxin level of 0.005 EU/ml. Then, the cells were resuspended in R10 media (RPMI 1640 supplemented with 10% heat-inactivated calf serum, 100U/ml penicillin G, 100 µl/ml streptomycin sulfate, and 1.7mM sodium glutamine) that contained 10U/ml DNase I (Roche Diagnostics, Mannheim, Germany) and rested for 1 hour before use. The cells were stimulated with 1ng/ml lipopolysaccharide (LPS; Toll-like receptor [TLR] 4 agonist) or 20ng/ml lipomannan from Mycobacterium smegmatis (LM-MS; TLR2 agonist), (both from InvivoGen, San Diego, CA) for in vitro stimulation or without stimuli for ex vivo results in the presence of 1 µg/ml of anti-CD28, 1 µg/ml of anti-CD49d (BD Biosciences), and 10 µg/ml of brefeldin A (Biolegend) at 37°C/5% CO2 for 6 hours. Surface staining was performed for 20 minutes at room temperature using the following: LIVE/DEAD fixable Violet Dead Cell Stain, CD8-Qdot605, CD14-Qdot655, and CD19-PB (Life Technologies); CD56-PB and CD16-PE-CF595 (BD Biosciences); and HLA-DR-BV570 (Biolegend). The cells were washed and permeabilized using a Cytofix/Cytoperm kit (BD Biosciences) and stained with the following conjugated antibodies for 20 minutes at room temperature: CD3-APC-H7, interleukin-6 (IL-6)-PE, IL1-α-FITC, and tumor necrosis factor-alpha (TNF-α)-Alexa700 (BD Biosciences); and IL-1β-Alexa647, IL-8-PerCP, and IL-10-PCy7 (Biolegend). The cells were fixed with 4% paraformaldehyde. Isotype controls for CD14 (Life Technologies); CD16, TNF-α, IL-6, and IL1-α (BD Biosciences); and IL-1β, IL-8, and IL-10 (Biolegend) were included in each experiment. Monocytes were defined as high Forward/Side scatter and expressed HLA-DR, CD14, and/or CD16 but not CD3, CD8, CD19, or CD56. The cells were analyzed using a LSRFortessa Cell Analyzer (BD Biosciences). A minimum of 2,000,000 total events and 20,000 monocytes were recorded in each tube.
Assay of Soluble Biomarkers and Anti-CMV Titers
Sera samples were collected in serum separation tubes, and plasma samples were collected in ethylene diamine tetra-acetic acid tubes. The levels of high-sensitivity CRP (hsCRP) and β2-microglobulin (β2M) were determined with an immunoturbidimetric sera assay using Cobas 701 (Roche Diagnostics, GmbH [Mannheim, Germany]). The D-dimer levels were measured with an automated latex enhanced immunoassay using plasma samples (HemosIL D-Dimer HS 500, Instrumentation Laboratory). The sera and plasma samples were aliquoted and stored at −20°C until subsequent analysis of the following biomarkers: IL-6, IL-8, IL-10, IL-1β, and TNF-α (R&D Systems, Minneapolis, MN), sCD14, (Diaclone, Besançon, France) as well as sCD163 (Macro CD163 IQproducts, The Netherlands). Additionally, anti-CMV immunoglobulin G (IgG) titers were assayed using a CMV IgG enzyme-linked immunosorbent assay (GenWay, San Diego, CA).
Clinical Tests
Cognitive decline and functional decline were assessed using the Mini-Mental State Examination (MMSE) (14) and the Barthel Index of Activities of Daily Living (15) score tests, respectively. These tests were determined by geriatricians (R.R. and M.I.G., respectively) who considered sensory and motor functioning. The researchers who conducted the laboratory determinations were blinded to the clinical data until the statistical analysis.
Statistical Analysis
Continuous variables are expressed as medians and interquartile ranges, and categorical variables are expressed as percentages. The correlation between the variables was assessed using the Spearman rank test. The Mann–Whitney U test was used to analyze differences between unpaired groups. Differences between paired samples were determined by the Wilcoxon signed rank test. All p values less than .05 were considered significant. Variables with a p value less than .1 in the univariate analysis were entered into a stepwise multivariate logistic regression model to determine the associations with anti-CMV IgG levels and the mini-mental test results after controlling for age, gender, CD4 and CD8 T-cell count, the CD4:CD8 ratio, the simple and multiple intracellular expression of cytokines ex vivo, and soluble inflammatory markers. Statistical analysis was performed using the Statistical Package for the Social Sciences software (SPSS 17.0; SPSS, Chicago, IL). Prism, version 5.0 (GraphPad Software, Inc.) was used for the generation of the graphs. We defined polyfunctionality as the percentage of monocytes that produce multiple cytokines. Polyfunctionality pie charts were constructed using Pestle, version 1.6.2 and Spice, version 5.2 (both provided by M. Roederer, NIH, Bethesda, MD) (16).
Results
Demographic, Hematologic, and Serologic Characteristics of the Study Populations
There were 20 elderly participants (9 men and 11 women, aged 83 [67–90] years) who lived at the Heliopolis nursing home (Seville, Spain) and 20 young volunteers as the control group (14 men and 6 women, aged 35 [27–40] years) enrolled in the study. The following results were obtained from the elderly and young control groups, respectively: the median CD4 T-cell count was 666 [416–984] vs 753 [583–1040] (cells/mm3); the CD8 T-cell count was 459 [305–812] vs 467 [308–619] (cells/mm3); the CD4:CD8 ratio was 1.4 [1–2.1] vs 1.7 [1.2–2.9]; and the levels of anti-CMV were 19.0 [9.9–27.2] vs 7.3 [0.0–18.2] (IU/mm3). No statistically differences were obtained in any of these variables, with the exception of the anti-CMV IgG levels, which were higher in the elderly group than in the young group (p = .009), as shown in Supplementary Figure 1. Of the 40 participants, 100% were considered CMV-seropositive (exhibiting signs of prior exposure) using the clinical cutoff point designated by the enzyme-linked immunosorbent assay test kits.
Monocyte Counts and the Frequency of the Activation of Phenotype Markers on Monocyte Subsets
The absolute total monocyte count was 455 [393–623] vs 330 [295–395] (cells/mm3), and the absolute classical monocyte count was 204 [167–276] vs 285 [205–377] (cells/mm3) in the elderly and young control groups, respectively. The total and classical absolute monocyte count was higher in the elderly group compared with that in the young group (p < .001, p = .024, respectively). We did not find differences in the frequency of monocyte subsets between groups. The percentage of activation and cell adhesion markers CD11b, CD40, CD49d, CD62L, CD163, and CD38 were similar in both groups regarding the three subsets. (Supplementary Table 1)
Intracellular Cytokine Production of Monocyte Subsets Ex Vivo and In Vitro Was Higher in the Elderly Group
Single cytokine production of monocytes
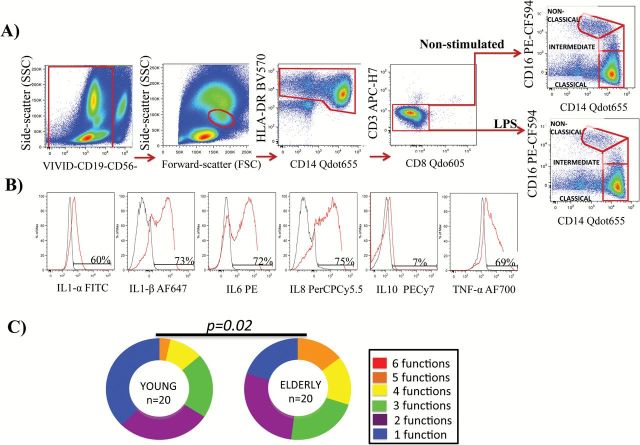
We addressed the characterization of the functionality, represented as IL1-α, IL1-β, IL-6, IL-8, IL-10, and TNF-α intracellular production ex vivo and responsiveness in vitro to LM-MS (TLR2 agonist) and LPS (TLR4 agonists) of the three monocyte subsets. Note that after LPS stimulation, despite the shedding of CD16, the nonclassical subset can be perfectly gated (Figure 1A and B).
Figure 1.
(A) Schematic diagram of the gating strategy of an elderly participant. Note that after LPS stimulation, despite the shedding of CD16, the nonclassical subset was gated. (B) Histogram representation of cytokine expression on classical monocytes without stimuli and with LPS stimulation. (C) Pie charts show classical monocytes with up to six functional responses to LPS stimulation. Statistically significant differences between the findings are shown. Pestle version 1.6.2 and Spice version 5.2 (M. Roederer, NIH, Bethesda, MD) were used.
Ex vivo, the elderly group showed a significantly higher percentage of intermediate monocytes that produced all of the cytokines described above. The results were similar for the other two subsets with the exception of IL10, whose levels did not differ between the subsets and TNF-α and whose levels were similar for classical monocytes in each group (Table 1 and Supplementary Figure 2). In vitro, the elderly group showed a significantly higher percentage of classical monocytes that expressed intracellular IL1-β and a higher percentage of the intermediate subset that expressed IL1-α, IL1-β, IL-8, and TNF-α in response to LM-MS. No significant differences were observed in the nonclassical subset in response to LM-MS (Table 1). Regarding LPS stimulation, although we did not observe significant differences between the groups, which was most likely due to the high dispersion of the values, it is important to note that the elderly group exhibited higher responsiveness in terms of almost all of the cytokines that showed twofold to threefold median increases compared with the young group in the three monocyte subsets.
Table 1.
Percentage of Each Monocyte Subset that Produces Different Intracellular Cytokines Ex Vivo and In Vitro
| Elderly Group (n = 20) | Young Group (n = 20) | p Values | p Values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ex Vivo | In Vitro | Ex Vivo | In Vitro | Ex Vivo | In Vitro | ||||
| LMMS | LPS | LMMS | LPS | LMMS | LPS | ||||
| Classical monocytes | |||||||||
| IL1-α | 0.36 (0.07–1.65) | 0.70 (0.09–3.42) | 7.35 (0.37–21.26) | 0.04 (0.02–0.11) | 0.09 (0.02–0.50) | 1.42 (0.62–6.59) | .001** | .060*** | .200 |
| IL1-β | 0.39 (0.19–1.45) | 8.00 (3.13–15.49) | 35.45 (18.89–54.20) | 0.13 (0.06–0.29) | 3.12 (1.26–5.82) | 13.45 (4.33–34.88) | .001** | .003** | .068*** |
| IL-6 | 0.59 (0.31–1.17) | 4.16 (2.50–8.97) | 23.35 (10.54–39.28) | 0.25 (0.10–0.47) | 3.05 (1.58–6.73) | 14.15 (4.47–34.53) | .002** | .140 | .240 |
| IL-8 | 2.26 (1.41–4.13) | 15.30 (8.19–27.94) | 44.19 (19.07–62.32) | 1.15 (0.49–1.62) | 12.63 (4.55–18.90) | 26.18 (11.85–58.81) | .007** | .080*** | .200 |
| IL-10 | 0.11 (0.04–0.50) | 0.03 (0.00–0.20) | 0.01 (0.00–1.33) | 0.18 (0.04–0.75) | 0.02 (0.00–076) | 0.05 (0.00–0.81) | .758 | .779 | .841 |
| TNF-α | 0.27 (0.14–0.42) | 1.44 (0.58–2.65) | 12.64 (4.14–25.52) | 0.20 (0.08–0.30) | 0.93 (0.39–1.94) | 6.80 (1.69–15.00) | .289 | .140 | .140 |
| Intermediate monocytes | |||||||||
| IL1-α | 1.91 (0.55–4.39) | 2.54 (0.37–5.49) | 15.54 (3.30–26.96) | 0.18 (0.04–0.40) | 0.19 (0.01–0.61) | 1.86 (0.50–7.87) | <.001* | .001** | .086*** |
| IL1-β | 1.37 (0.57–3.85) | 9.29 (4.00–15.08) | 32.71 (16.84–46.76) | 0.56 (0.18–0.78) | 2.86 (1.48–8.70) | 10.56 (3.25–29.67) | .004** | .007** | .072*** |
| IL-6 | 1.28 (0.67–2.67) | 5.86 (3.69–11.42) | 24.55 (8.89–40.95) | 0.62 (0.22–1.61) | 4.45 (1.88–7.80) | 11.00 (4.76–21.62) | .021** | .211 | .192 |
| IL-8 | 4.42 (1.99–7.58) | 14.24 (10.94–24.72) | 36.35 (15.88–51.62) | 0.77 (0.43–3.86) | 10.83 (2.63–18.52) | 21.44 (7.18–37.25) | .003** | .049** | .157 |
| IL-10 | 2.10 (0.58–4.39) | 0.10 (0.00–1.69) | 0.14 (0.00–1.59) | 0.41 (0.06–1.56) | 0.05 (0.00–0.78) | 0.11 (0.00–1.64) | .004** | .738 | .904 |
| TNF-α | 0.57 (0.39–1.07) | 3.14 (1.33–5.54) | 13.02 (2.75–29.16) | 0.30 (0.12–0.61) | 0.89 (0.53–2.65) | 4.49 (1.94–14.00) | .026** | .030** | .183 |
| Nonclassical monocytes | |||||||||
| IL1-α | 1.10 (0.56–2.12) | 0.33 (0.00–2.00) | 0.48 (0.00–0.88) | 0.31 (0.15–0.55) | 0.35 (0.00–1.06) | 0.30 (0.00–1.55) | <.001* | .841 | .947 |
| IL1-β | 1.52 (0.75–3.90) | 1.73 (0.51–4.97) | 1.69 (0.00–5.00) | 0.50 (0.18–1.22) | 1.05 (0.50–3.43) | 1.10 (0.16–1.93) | .001** | .565 | .738 |
| IL-6 | 0.87 (0.23–1.83) | 0.81 (0.08–2.58) | 0.92 (0.11–3.03) | 0.23 (0.08–0.83) | 0.31 (0.00–1.24) | 0.16 (0.02–1.62) | .068*** | .341 | .192 |
| IL-8 | 6.81 (2.35–11.78) | 1.19 (0.00–4.21) | 1.70 (0.16–6.35) | 1.45 (0.70–6.67) | 1.23 (0.37–4.29) | 3.45 (0.32–6.38) | .038** | .583 | .565 |
| IL-10 | 4.21 (1.05–9.97) | 0.77 (0.00–2.08) | 0.00 (0.00–1.63) | 2.37 (1.04–5.16) | 0.40 (0.14–2.24) | 0.60 (0.00–1.96) | .327 | .678 | .211 |
| TNF-α | 2.43 (1.14–3.91) | 1.67 (0.23–3.31) | 1.56 (0.18–3.60) | 1.12 (0.54–1.63) | 1.17 (0.32–4.78) | 1.03 (0.22–4.04) | .010** | .883 | .925 |
Notes: Values are expressed as the median and interquartile range. Mann–Whitney U test was used to compare the values of the elderly group with those of the the young control group.
*p < .001. **p < .05. ***p < .1.
Multiple Cytokine Production of Monocytes
We also analyzed the characterization of simultaneous multiple cytokine expression per cell, which is also termed polyfunctionality. The classical subset showed higher polyfunctionality distribution ex vivo in the elderly participants (data not shown). After stimulation with LPS, the classical monocytes in the elderly group expressed significantly higher levels of multiple cytokines compared with the young group (Figure 1C). No differences were observed for the other monocyte subsets either after LM-MS stimulation or based on the ex vivo results. Nonetheless, a more in-depth analysis of the 63 possible multiple cytokine combinations ex vivo revealed that the specific proinflammatory cytokine combination of cells expressing IL1-α+, IL1-β+, IL-6+, IL-8+, IL-10−, and TNF-α+ (a+b+6+8+10-T+) was higher in the classical and intermediate monocytes of the elderly group (p = .014 and p = .015, respectively). Additionally, the elderly group showed a higher percentage of nonclassical monocytes that simultaneously produced IL1-α+, IL1-β+, IL-6−, IL-8+, IL-10+, and TNF-α+ (a+b+6–8+10+T+; p = .048). Significantly more combination of four, three, four, and five cytokines occurred in the three monocyte subsets in the elderly group (data not shown). The detailed analysis of the in vitro responsiveness revealed similar results; the classical and intermediate monocytes exhibited a greater polyfunctional proinflammatory response to LM-MS and LPS (data not shown).
Single and Multiple Intracellular Functionality of Monocytes Is Associated With Higher Soluble Levels of Inflammatory and Coagulation Biomarkers
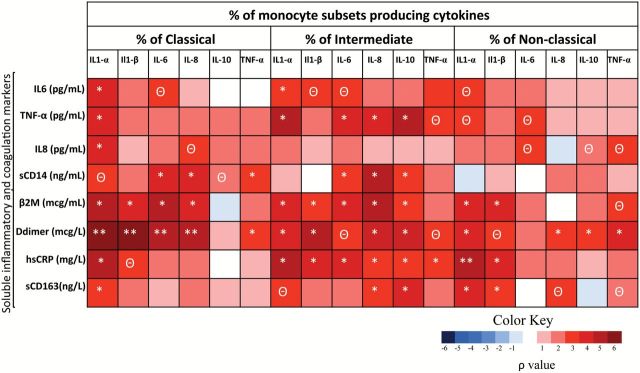
We determined the soluble concentrations of inflammatory and coagulation biomarkers. The IL-1β and IL-10 levels were below the detection limit of the assay. Consistent with previous data (1), the elderly group exhibited higher soluble levels of IL-8, IL-6, TNF-α, hsCRP, β2M, and D-dimer compared with the young control group (Supplementary Table 2). Interestingly, we found that the ex vivo single cytokine production was positively and strongly correlated with soluble inflammatory levels in the three monocyte subsets. Remarkably, the β2M and D-dimer levels were strongly and positively associated with the expression of almost all of the intracellular cytokines in the three monocyte subsets (Figure 2). When this analysis was applied to multiple cytokine combinations, in the classical subset, the ex vivo polyfunctionality was positively correlated with the soluble inflammatory markers, especially with β2M and D-dimer (Supplementary Figure 3). Similar results were obtained for the intermediate and nonclassical subset (data not shown).
Figure 2.
Correlations among the soluble biomarkers and monocyte function ex vivo in the elderly and young groups (n = 40). Heatmap representing negative (blue shading) and positive (red shading) associations between soluble inflammatory markers and single intracellular cytokine production in the three monocyte subsets. Spearman ρ correlation coefficient test was used. **p < .001. *p < .05.Θ p < .1.
Regarding the phenotype activation markers, we also found strong associations between activation cell surface markers on monocytes and soluble inflammatory concentrations (data not shown). Of special interest were the associations of the scavenger receptor CD163 with the following in the elderly group: sCD14 (p = .045; ρ = .465), IL-6 (p = .020; ρ = .542), hsCRP (p = .029; ρ = .500), and β2M (p = .004; ρ = .634).
Monocyte Activation Phenotype Is Associated With Anti-CMV Titers and Functional and Cognitive Decline in the Elderly Group
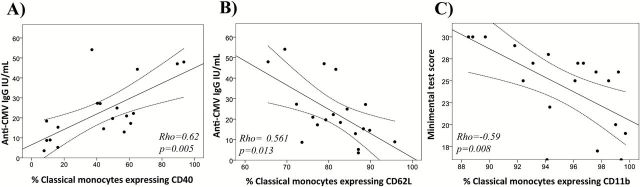
The relationship between monocyte activation and anti-CMV IgG antibodies in the elderly group was analyzed. Soluble CD163 was positively associated with anti-CMV IgG levels (p = .007, ρ = .579) in the elderly group. Regarding surface expression, CD62L in the classical subset was inversely and independently associated with anti-CMV IgG levels (Table 2) after adjusting for age, gender, CD4 and CD8 T-cell count, the CD4:CD8 ratio, the ex vivo single and multiple intracellular production of cytokines, and soluble inflammatory markers. Figure 3A and B shows the graphical representation of the correlations between anti-CMV IgG levels and CD40 (p = .005, ρ = .618) and CD62L (p = .013, ρ = −.561) surface expression on classical monocytes. On the contrary, there was no association between those parameters in the young group.
Table 2.
Relationship Between Immunological Variables and Monocyte Markers With Anti-CMV IgG Levels in the Elderly Group
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| β | CI (95%) | p Value | β | CI (95%) | p Value | |
| % CD11b expression on classical | 1.779 | −0.127 to 3.685 | .065 | NS | ||
| % CD40 expression on classical | 0.385 | 0.169 to 0.601 | .002 | NS | ||
| % CD62L expression on classical | −1.179 | −1.97 to −0.387 | .006 | −1.477 | −2.256 to −0.698 | .001 |
| CD4:CD8 | −7.56 | −16.193 to −1.073 | .082 | NS | ||
Note: CI = confidence interval; NS = Nonsignificant. Stepwise regression model.
Figure 3.
(A and B) Graphical representation of the relationship between anti-cytomegalovirus titers and the percentage of monocytes expressing CD40 and CD62L. (C) Graphical representation of the association between Mini-Mental State Examination and the CD11b expression on classical monocytes in the elderly group. Spearman ρ correlation coefficient test was used.
We were interested in analyzing which factors are associated with the functional and cognitive decline. According to the Mahoney and Barthel definition of the test, we categorized the highest Barthel score (100), which means that the person requires no assistance with any aspect of the tasks of the test as functional independence (n = 11/20). If the Barthel score was below 100, then we categorized the person as no functional independence (n = 9/20). According to these groups, we found that the CD163 and CD38 surface expression on classical monocytes was higher in the functional independence group (p = .041, p = .026, respectively). There were no differences in gender, CD4 and CD8 T-cell count, CD4:CD8 ratio or anti-CMV IgG levels between the categories. We also examined the relationship of the immunological variables and monocyte markers with the cognitive decline assessed with the MMSE test. Only the expression of CD11b in the classical subset and the CD4:CD8 ratio were independently associated with the MMSE (Table 3) results after adjusting for age, gender, CD4 and CD8 T-cell count, the CD4:CD8 ratio, the ex vivo single and multiple intracellular production of cytokines, and the soluble inflammatory markers. Figure 3C shows the graphical representation of the correlations between the MMSE and the CD11b expression on classical monocytes in the elderly group.
Table 3.
Relationship Between Immunological Variables and Monocyte Markers With Mini-mental Test Score in the Elderly Group
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| β | CI (95%) | p Value | β | CI (95%) | p Value | |
| % CD11b expression on classical | −0.815 | −1.34 to −0.29 | .004 | −0.888 | −1.42 to −0.35 | .003 |
| Age | −0.213 | −0.38 to −0.046 | .015 | NS | ||
| % CD40 expression on classical | −0.083 | −0.17 to −0.002 | .046 | NS | ||
| % CD62L expression on classical | 0.291 | 0.014 to 0.567 | .041 | NS | ||
| Anti-CMV IgG | −0.132 | −0.27 to 0.012 | .070 | NS | ||
| CD4:CD8 | 3.807 | 1.493–6.121 | .003 | 2.769 | 0.873–4.666 | .007 |
Note: CI = confidence interval. Stepwise regression model.
Discussion
The results presented herein comprehensively characterize a higher single and multiple intracellular cytokine production by monocytes both ex vivo and in vitro in the elderly group. Moreover, the ex vivo cytokine expression together with phenotype activation of surface markers of monocytes was strongly associated with soluble inflammatory biomarkers, such as IL-6, D-dimer, hsCRP, and sCD14, which are all known to be associated with increased morbidity risk. In addition to these findings, we showed that phenotype activation markers of monocytes are associated with anti-CMV IgG levels and with the functional and cognitive decline in the elderly group.
The present study adds novel data to the hyperactivation and functional deregulation of monocytes with aging. We carefully characterized the intracellular cytokine responsiveness ex vivo and to TLR-2 and TLR-4 agonists in vitro and showed that the responsiveness was higher in the elderly group. The nonclassical subset showed the lowest responsiveness to TLR-2 and TLR-4 stimulation according to previous data (8). In addition, ex vivo, this subset expressed the highest levels of TNF-α and IL-10, and these data support the evidence that the three monocyte subsets are not only phenotypically different but also functionally different.
We also characterized the polyfunctionality changes of these cells with advanced age. We interestingly found that multiple cytokine distribution was higher in the classical subset ex vivo and in response to LPS with aging. Most importantly, the expression of both single and combinations of multiple intracellular cytokines ex vivo was strongly associated with soluble inflammation and coagulation biomarkers, which suggests that monocytes are the key cell source of the chronic inflammatory state defined in aging. In the T-cell scenario, immunosenescence is related to the changing phenotypic composition of T cells with age. Impaired T-cell polyfunctionality in older adults have been associated with an increased 2-year mortality rate (17) and leads to diminished immune responses following vaccination (18). In the HIV field, enhanced polyfunctionality in HIV-1–specific CD8+ T cells has been associated with superior control of the virus (19). Our results demonstrate that monocyte polyfunctionality in the elderly group is not desirable because it is correlated with soluble inflammatory markers that are known to be associated with increased morbidity and mortality risk.
Mortality and morbidity in the elderly group are associated with impaired innate immunity to pathogens, CMV infection, and a persistent low-grade soluble inflammatory state (2,5,20). The present findings are the first to demonstrate the relationship between monocyte activation, anti-CMV IgG titers, and functional and cognitive decline in the aged population. CD62L is an adhesion molecule that is responsible for monocyte rolling and adhesion to endothelial cells. We found that the expression of CD62L on classical monocytes was strongly and inversely associated with anti-CMV IgG levels, which is even more consistent and profound than the CD4:CD8 ratio, a well-established marker of immunosenescence. The reduced surface expression of CD62L on monocytes with aging has been described by other authors (21). The downregulation of CD62L is believed to impair rolling and may increase firm attachment of the cells to vessels, favoring endothelial migration. This mechanism may therefore promote atherosclerotic plaque formation and other inflammatory diseases in the elderly population.
Cognitive and functional impairments are important causes of increased morbidity and mortality in older people. Researchers agree that understanding the mechanisms to establish early diagnosis and treatment benefits is critical. We found that the phenotype activation of monocytes is strongly associated with functional and cognitive decline in the elderly group. Our results provide a more complete understanding of the mechanisms that contribute to the neuropathogenesis of age-related diseases. Moreover, we showed that the expression of the CD11b integrin, which pairs with CD18 to form a heterodimeric type 1 transmembrane receptor (CD11b/CD18; β2αM) on classical monocytes (an easily accessible biomarker), was strongly associated with the rate of cognitive decline in the elderly group, in addition to the currently used T-cell markers. Recently, the upregulation of other cell adhesion surface markers, such as the chemokine receptor 2 (CCR2) on CD16+ monocytes, has been associated with HIV-associated neurocognitive disorders in the premature immunosenescence of HIV-infected patients (22). Our results provide more evidence that the activation of monocytes may significantly contribute to the development of age-related diseases.
The potential mechanisms that drive these phenotypes of the hyperactivation and high inflammatory ex vivo and in vitro state of monocytes could involve persistent viral infections such as CMV that stimulate and exhaust the immune system. CMV is thought to persist in a latent state with its DNA genome harbored primarily in monocytes (23), which establishes a chronic infection with intermittent reactivations. CMV reactivations and new reinfections that occur more frequently in the elderly group (24) than in the young might be a driving force in the inflammatory state and the activation of monocytes/macrophages (11,12). In vitro studies demonstrated that upon infection with other viruses, these cells mediated the differentiation of resting B cells to plasmablasts as well as IgG antibodies via BAFF/APRIL and IL-10 signaling (25). Hence, this could be the linking mechanism between monocyte activation and the demonstrated enhanced anti-CMV IgG levels. However, we cannot exclude other possible causes such as microbial translocation caused by barrier defects in gut-associated lymphoid tissue related to advanced age, reactivation of endogenous pathogens such as Mycobacterium tuberculosis and other herpesviruses.
Our study was limited by the small numbers of participants, and we did not have sufficient power to statistically assess the relationship between the enhanced CD11b expression on monocytes for the diagnoses of cognitive impairment and the decreased CD38 and CD163 expression on monocytes for the diagnoses of functional impairment. CD163 is a member of a scavenger receptor super family and is a specific monocyte and macrophage activation marker. The shedding upon activation of these cells leads to the reduced CD163 surface expression, and the higher levels of the soluble form of CD163 have been associated with age-associated diseases (26,27). Future studies with larger cohorts will be performed to more clearly evaluate the associations among phenotype changes and worse clinical outcomes. Other limitation of our data is the fact that all young controls were CMV seropositive, this makes impossible to analyze separately the effect of age and CMV infection in our study. In our previous work (28), we described 82% prevalence of CMV seropositivity in young controls with median age of 33 years, 2 years younger than the median age in this work, these discrepancies may be due to different sensitivity of the kits used for determinations. Additionally, more in vitro and in vivo longitudinal studies should be performed to analyze the underlying mechanisms in more detail and to develop approaches for determining the cause and effect relationship among CMV infection, the age-related monocyte changes, and clinical impairment.
In summary, our results demonstrate that monocytes are key cell candidates for the potential source of the high soluble inflammatory levels of aged participants. Our findings suggest that CMV infection might be a driving force in the activation of monocytes shown to be associated with the functional and cognitive decline in the elderly group.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This study was supported by Redes Tematicas de Investigacion en SIDA (ISCIII RETIC RD12/0017/0029 and RD06/0006/0035), Proyecto de Excelencia, Consejeria de Innovacion, Ciencia y Empresa (P11-CTS-06313), and Consejeria Andaluza de Salud (PI-0278-2010). E.R.-M received grants from (Instituto de Salud Carlos III CPII014/00025). This work was supported in part by Ramon y Cajal grant from Ministry of Economy and Competitiveness RYC-2010–07419 to M.R.B.
Supplementary Material
Acknowledgments
The authors express their most sincere thanks to all of the volunteers of the HELIOPOLIS nursing home and the young control volunteers. We are grateful to Magdalena Rodriguez, Marien Gutierrez Sancho, and Ana Maria Guijarro from the Day Care Hospital (Infectious Diseases Department) for their outstanding assistance. R.P.-B., S.F.-M., E.R.-M., and M.L. designed the study. R.P.-B. produced the experimental data in the laboratory. R.R., J.C., and M.I.G. determined the clinical test. R.P.-B. analyzed the data and prepared the manuscript with critical review from all of the authors.
References
- 1. Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci. 2000;908:244–254. doi:10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- 2. Holmes C, Cunningham C, Zotova E, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–774. doi:10.1212/WNL.0b013e3181b6bb95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. doi:10.1161/01.CIR.0000097109.90783.FC [DOI] [PubMed] [Google Scholar]
- 4. Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol. 2010;172:363–371. doi:10.1093/aje/kwq177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aiello AE, Haan M, Blythe L, Moore K, Gonzalez JM, Jagust W. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54:1046–1054. doi:10.1111/j.1532-5415.2006.00796.x [DOI] [PubMed] [Google Scholar]
- 6. Wikby A, Ferguson F, Forsey R, et al. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci. 2005;60:556–565. doi:10.1093/gerona/60.5.556 [DOI] [PubMed] [Google Scholar]
- 7. Hearps AC, Martin GE, Angelovich TA, et al. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell. 2012;11:867–875. doi:10.1111/j.1474-9726.2012.00851.x [DOI] [PubMed] [Google Scholar]
- 8. Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi:10.1016/j.immuni.2010.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Itzhaki RF, Wozniak MA, Appelt DM, Balin BJ. Infiltration of the brain by pathogens causes Alzheimer’s disease. Neurobiol Aging. 2004;25:619–627. doi:10.1016/j.neurobiolaging.2003.12.021 [DOI] [PubMed] [Google Scholar]
- 10. Ringheim GE, Conant K. Neurodegenerative disease and the neuroimmune axis (Alzheimer’s and Parkinson’s disease, and viral infections). J Neuroimmunol. 2004;147:43–49. doi:10.1016/j.jneuroim.2003.10.013 [DOI] [PubMed] [Google Scholar]
- 11. Yew KH, Carpenter C, Duncan RS, Harrison CJ. Human cytomegalovirus induces TLR4 signaling components in monocytes altering TIRAP, TRAM and downstream interferon-beta and TNF-alpha expression. PLoS One. 2012;7:e44500. doi:10.1371/journal.pone.0044500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith PD, Shimamura M, Musgrove LC, et al. Cytomegalovirus enhances macrophage TLR expression and MyD88-mediated signal transduction to potentiate inducible inflammatory responses. J Immunol. 2014;193:5604–5612. doi:10.4049/jimmunol.1302608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saederup N, Cardona AE, Croft K, et al. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5:e13693. doi:10.1371/journal.pone.0013693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 15. Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 16. Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79:167–174. doi:10.1002/cyto.a.21015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferrando-Martínez S, Ruiz-Mateos E, Casazza JP, et al. IFNγ–TNFα–IL2–MIP1α–CD107a+PRF1+ CD8 pp65-specific T-cell response is independently associated with time to death in elderly humans. J Gerontol A Biol Sci Med Sci. 2014. pii:glu171. doi:10.1093/gerona/glu171. [Epub ahead of print].. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172:3437–3446. doi:10.4049/jimmunol.172.6.3437 [DOI] [PubMed] [Google Scholar]
- 19. Almeida JR, Price DA, Papagno L, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–2485. doi:10.1084/jem.20070784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verschoor CP, Johnstone J, Millar J, et al. Alterations to the frequency and function of peripheral blood monocytes and associations with chronic disease in the advanced-age, frail elderly. PLoS One. 2014;9:e104522. doi:10.1371/journal.pone.0104522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Martinis M, Modesti M, Ginaldi L. Phenotypic and functional changes of circulating monocytes and polymorphonuclear leucocytes from elderly persons. Immunol Cell Biol. 2004;82:415–420. doi:10.1111/j.0818-9641.2004.01242.x [DOI] [PubMed] [Google Scholar]
- 22. Williams DW, Byrd D, Rubin LH, Anastos K, Morgello S, Berman JW. CCR2 on CD14(+)CD16(+) monocytes is a biomarker of HIV-associated neurocognitive disorders. Neurol Neuroimmunol Neuroinflamm. 2014;1:e36. doi:10.1212/NXI.0000000000000036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Minton EJ, Tysoe C, Sinclair JH, Sissons JG. Human cytomegalovirus infection of the monocyte/macrophage lineage in bone marrow. J Virol. 1994;68:4017–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, Glaser R. Chronic herpesvirus reactivation occurs in aging. Exp Gerontol. 2007;42:563–570. doi:10.1016/j.exger.2007.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kwissa M, Nakaya HI, Onlamoon N, et al. Dengue virus infection induces expansion of a CD14(+)CD16(+) monocyte population that stimulates plasmablast differentiation. Cell Host Microbe. 2014;16:115–127. doi:10.1016/j.chom.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greisen SR, Moller HJ, Stengaard-Pedersen K, et al. Soluble macrophage-derived CD163 is a marker of disease activity and progression in early rheumatoid arthritis. Clin Exp Rheumatol. 2011;29:689–692. [PubMed] [Google Scholar]
- 27. Aristoteli LP, Møller HJ, Bailey B, Moestrup SK, Kritharides L. The monocytic lineage specific soluble CD163 is a plasma marker of coronary atherosclerosis. Atherosclerosis. 2006;184:342–347. doi:10.1016/j.atherosclerosis.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 28. De Pablo-Bernal RS, Ruiz-Mateos E, Rosado I, et al. TNF-α levels in HIV-infected patients after long-term suppressive cART persist as high as in elderly, HIV-uninfected subjects. J Antimicrob Chemother. 2014;69:3041–3046. doi:10.1093/jac/dku263 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.