Table 1.
Synthetic library of β-nitrostyrenes.
| Compound | Basic moiety | R | R' | R“ | Yieldsa (%) | M.P.(°C) | M.P.reference |
|---|---|---|---|---|---|---|---|
| 1 | 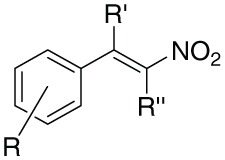 |
4-Cl | H | H | 81 | 112–114 | 113–114 |
| 2 | 4-Cl | H | Me | 82 | 88–90 | 89–91 | |
| 3 | 4-Cl | H | Et | 80 | 105–107 | ||
| 4 | 4-NMe2 | H | H | 91 | 186–188 | 186–188 | |
| 5 | 4-NMe2 | H | Me | 96 | 90–91 | ||
| 6 | 4-NMe2 | H | Et | 92 | 77–79 | ||
| 7 | 4-NO2 | H | H | 90 | 94–96 | 94–96 | |
| 8 | 4-NO2 | H | Me | 89 | 114–116 | 114–115 | |
| 9 | 4-NO2 | H | Et | 81 | 104–106 | 103.5–104.5 | |
| 10 | 3-NO2 | H | H | 79 | 124–126 | 125 | |
| 11 | 3-NO2 | H | Me | 81 | 80–82 | ||
| 12 | 3-NO2 | H | Et | 84 | 84–86 | ||
| 13 | 4-OMe | H | H | 89 | 86–88 | 85–87 | |
| 14 | 4-OMe | H | Me | 88 | 44–46 | 44–45 | |
| 15 | 4-OMe | H | Et | 73 | Oil | Oil | |
| 16 | 4-OH | H | H | 76 | 168–169 | 167–171b | |
| 17 | 4-OH | H | Me | 72 | 122–124 | 124–125 | |
| 18 | 4-OH | H | Et | 71 | 60–62 | ||
| 19 | 3-OH | H | H | 79 | 138–140 | 136–140b | |
| 20 | 3-OH | H | Me | 81 | 96–98 | 96–98 | |
| 21 | 3-OH | H | Et | 79 | 82–84 | ||
| 22 | 3,4-dimethoxy | H | H | 88 | 140–142 | 142–144 | |
| 23 | 3,4-dimethoxy | H | Me | 89 | 70–72 | 71–72 | |
| 24 | 3,4-dimethoxy | H | Et | 91 | 78–80 | 78–79 | |
| 25 | 4-OH | Me | H | 77 | 88–90 | ||
| 26 | 3-OH | Me | Et | 74 | 138–140 | ||
| 27 | 2-OH,3-OEt | H | H | 87 | 128–130 | ||
| 28 | 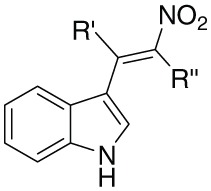 |
- | H | H | 89 | 134–136 | |
| 29 | - | H | Me | 91 | 182–184 | ||
| 30 | - | H | Et | 86 | 64–66 |
Isolated yields.
bMelting points of compounds from sigma aldrich.
