Abstract
The taxonomy of the synnematous genera Cephalotrichum, Doratomyces and Trichurus, and other related genera Gamsia, Wardomyces and Wardomycopsis, has been controversial and relies mainly on morphological criteria. These are microascaceous saprobic fungi mostly found in air and soil and with a worldwide distribution. In order to clarify their taxonomy and to delineate generic boundaries within the Microascaceae, we studied 57 isolates that include clinical, environmental and all the available ex-type strains of a large set of species by means of morphological, physiological and molecular phylogenetic analyses using DNA sequence data of four loci (the ITS region, and fragments of rDNA LSU, translation elongation factor 1α and β-tubulin). The results demonstrate that Cephalotrichum, Doratomyces and Trichurus are congeneric and the genus Cephalotrichum is accepted here with Echinobotryum as a further synonym. The genera Acaulium and Fairmania, typified by A. albonigrescens and F. singularis, respectively, are distinct from Microascus and Scopulariopsis, Gamsia is distinct from Wardomyces, and Wardomycopsis is confirmed as a separate genus in the Microascaceae. Two new species of Cephalotrichum are described as C. brevistipitatum and C. hinnuleum. Nine new combinations are proposed, i.e. Acaulium acremonium, A. caviariforme, Cephalotrichum asperulum, C. columnare, C. cylindricum, C. dendrocephalum, C. gorgonifer, Gamsia columbina and Wardomyces giganteus. A neotype is designed for C. stemonitis. Lectotypes and epitypes are designated for A. acremonium, A. albonigrescens, C. gorgonifer, C. nanum and W. anomalus. Cephalotrichum cylindricum, C. microsporum, F. singularis and Gamsia columbina are also epitypified with new specimens. Descriptions of the phenotypic features and dichotomous keys for identification are provided for accepted species in the different genera.
Key words: Cephalotrichum, Doratomyces, Gamsia, Trichurus, Wardomyces, Wardomycopsis, Microascales, Multigene phylogeny, Taxonomy
Taxonomic novelties: New species: Cephalotrichum brevistipitatum Sandoval-Denis, Guarro & Gené; Cephalotrichum hinnuleum Sandoval-Denis, Guarro & Gené
New combinations: Acaulium acremonium (Delacr.) Sandoval-Denis, Guarro & Gené; Acaulium caviariforme (Malloch & Hubart) Sandoval-Denis, Guarro & Gené; Cephalotrichum asperulum (J.E. Wright & S. Marchand) Sandoval-Denis, Guarro & Gené; Cephalotrichum columnare (H.J. Swart) S.P. Abbott; Cephalotrichum cylindricum (Clem. & Shear) S. P. Abbott; Cephalotrichum dendrocephalum (Udagawa, Y. Horie & Abdullah) S.P. Abbott; Cephalotrichum gorgonifer (Bainier) Sandoval-Denis, Gené & Guarro; Gamsia columbina (Demelius) Sandoval-Denis, Guarro & Gené; Wardomyces giganteus (Malloch) Sandoval-Denis, Guarro & Gené
Typification: Epitypification (basionyms): Acaulium albonigrescens Sopp, Fairmania singularis Sacc., Monilia acremonium Delacr., Periconia nana Ehrenb., Stysanus microsporus Sacc., Trichurus cylindricus Clem. & Shear, Trichurus gorgonifer Bainier, Trichosporum columbinum Demelius, Wardomyces anomalus F.T. Brooks & Hansf.
Lectotypification (basionyms): Acaulium albonigrescens Sopp, Monilia acremonium Delacr., Periconia nana Ehrenb., Trichurus gorgonifer Bainier, Wardomyces anomalus F.T. Brooks & Hansf.
Neotypification: Isaria stemonitis Pers.: Fr.
Introduction
The family Microascaceae, as established by Luttrell (Malloch 1970a), currently accommodates a morphologically heterogeneous group of fungi, comprising saprobic and plant pathogenic species. Some species of Microascaceae are opportunistic pathogens of humans and show intrinsic resistance to antifungal agents (Hoog et al., 2011, Sandoval-Denis et al., 2013, Sandoval-Denis et al., 2016, Lackner et al., 2014).
Recent molecular studies have demonstrated that the Microascaceae contains several closely related genera that are difficult to separate morphologically (Sandoval-Denis et al. 2016). Members of the family are characterised by the presence of mostly annellidic asexual morphs with dry aseptate conidia and by sexual morphs that form cleistothecial or perithecial, carbonaceous ascomata producing reniform, lunate or triangular ascospores with or without germ pores. The most studied genera are Microascus, Scedosporium and Scopulariopsis, primarily because of their clinical importance (Sandoval-Denis et al., 2013, Lackner et al., 2014). Lackner et al. (2014) delimited phylogenetic boundaries among genera of the Scedosporium clade by means of 28S large subunit (LSU) and internal transcribed spacer (ITS) sequence analyses. Recently, three of the most debated genera of the family, Microascus, Scopulariopsis and Pithoascus were revised through a detailed morphological study combined with a four-gene phylogeny (Sandoval-Denis et al. 2016). As a result, several taxa were excluded from these genera and still remain in an uncertain taxonomic position. For instance, the genus Acaulium, previously considered a synonym of Scopulariopsis was suggested to be a distinct genus, while Microascus singularis, the type species of the genus Fairmania, currently a synonym of Microascus (Curzi, 1931, Barron et al., 1961, Udagawa and Awao, 1969, Arx et al., 1988), appeared as a new lineage within the Microascaceae (Sandoval-Denis et al. 2016). Furthermore, the phylogeny and taxonomy of several lesser-known genera of the Microascaceae are still unresolved. Current concepts of the synnematous genera Cephalotrichum, Doratomyces and Trichurus, and the related genera Gamsia, Wardomyces and Wardomycopsis, having conidia with germ slits, are based exclusively on morphological criteria. Ex-type cultures are unavailable for several species of these genera and DNA sequences are scarce or of doubtful quality.
Cephalotrichum Link (1809) is tied to C. stemonitis (formerly Periconia stemonitis) after it was lectotypified by Hughes (1958). It is characterised by the production of dry conidia in basipetal chains from percurrently extending (annellidic) conidiogenous cells that arise on the upper part of large dark pigmented synnemata (Abbott 2000). According to Index Fungorum Cephalotrichum currently comprises 68 species, 25 of them of uncertain application. Doratomyces (Sturm 1829), typified by D. neesii, currently includes 22 species, five of them of uncertain application and shares morphological characteristics with Cephalotrichum. Since the application of Cephalotrichum was unclear to them, Morton & Smith (1963) considered the former as a possible synonym of Doratomyces whereas other authors, following the lectotypification of the genus by Hughes (1958), considered Cephalotrichum as the correct name for this genus (Carmichael et al., 1980, Arx, 1981, Abbott, 2000, Jiang et al., 2011, Seifert et al., 2011, Beer et al., 2013). Cephalotrichum was sanctioned by Fries (1832), and it is currently included in the proposed List of Protected Fungal Generic Names (Kirk et al. 2013). Trichurus (Clements 1896) is typified by T. cylindricus and currently comprises five species. It is also morphologically similar to Cephalotrichum and Doratomyces, but distinguished by the presence of setae on the upper part of the synnemata (Morton & Smith 1963). However, detailed ultrastructural studies on the synnematal morphogenesis suggested that the sole presence of setae might not support their distinction as a different genus (Hasselbring, 1896, Swart, 1964, Hammill, 1977, Abbott, 2000). Abbott (2000) studied a large set of strains belonging to these fungi and concluded that the three genera were congeneric, which led to numerous proposed new combinations, but they were not formally published.
Wardomyces (W.), typified by W. anomalus, is characterised by polyblastic conidiogenous cells borne on undifferentiated hyphae and dark, 0–1-septate conidia with characteristic longitudinal germ slits. The generic concept was expanded with the inclusion of W. columbinus, showing a secondary type of conidia formed on annellides (Hennebert 1968) and with the addition of W. aggregatus, W. dimerus and W. simplex, all having septate annelloconidia (Gams, 1968, Sugiyama et al., 1968, Malloch, 1970b). Wardomyces columbinus and W. ovalis were transferred to the genus Hennebertia and W. dimerus to Gamsia, typified with G. dimera (Morelet 1969), but these transfers were not widely accepted (Domsch et al., 2007, Seifert et al., 2011, Whitton et al., 2012). Although lacking synnemata, Wardomyces has been shown to be phylogenetically related to Cephalotrichum (Issakainen et al., 2003, Lackner et al., 2014, Sandoval-Denis et al., 2016). Wardomycopsis (Ws.), typified by Ws. inopinata (Udagawa & Furuya 1978), is similar to Wardomyces in the presence of dark conidia with longitudinal germ slits, but differs in that the conidia are borne on annellidic conidiogenous cells and are arranged in short chains. Its phylogenetic position is still unresolved, although it has been shown that Wardomycopsis species cluster as a distinct and well-supported lineage within the Microascaceae (Sandoval-Denis et al. 2016).
In this study a polyphasic approach is carried out, using phenotypic features and DNA sequence data for all available living type material and several authentic and reference strains from public collections, to resolve the taxonomy of main genera of Microascaceae including Cephalotrichum, Doratomyces and Trichurus, characterised by the production of synnemata, and Gamsia, Wardomyces and Wardomycopsis characterised by dark conidia with germ slits.
Materials and methods
Isolates
Fifty-six isolates belonging to 29 species of Cephalotrichum, Doratomyces, Gamsia, Microascus, Scopulariopsis, Trichurus, Wardomyces and Wardomycopsis were examined, including all the available ex-type cultures for the mentioned species. Type material was obtained from the collections of the CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands (CBS); Facultat de Medicina, Universitat Rovira I Virgili, Reus, Spain (FMR), Institute of Hygiene and Epidemiology-Mycology Laboratory, Brussels, Belgium (IHEM), UAMH Centre for Global Microfungal Biodiversity, University of Toronto, Canada (UAMH) and the University of Texas Health Science Center at San Antonio, Texas, USA (UTHSCSA) (Table 1), and from different herbaria for which acronyms are listed according to Index Herbariorum (http://sweetgum.nybg.org/science/ih/).
Table 1.
Strains and sequence accession numbers included in this study.
| Current name | Original name | Strain number1 | Source2 | Origin | Sequence accession number3 |
|||
|---|---|---|---|---|---|---|---|---|
| LSU | ITS | TEF | TUB | |||||
| Acaulium acremonium | Scopulariopsis danica | CBS 290.38 (Ex-type) | Skin of a horse | Denmark | LN851001 | LM652456 | HG380362 | LN851108 |
| Scopulariopsis acremonium | MUCL 8274 (Ex-epitype) | Wheat field soil | Germany: Schleswig-Holstein | LN851002 | LM652457 | LN851056 | LN851109 | |
| Scopulariopsis acremonium | MUCL 8409 | Soil | Germany: Schleswig-Holstein | LN851003 | LM652458 | LN851057 | LN851110 | |
| A.albonigrescens | Microascus albonigrescens | IHEM 18560 (Ex-epitype) | Litter treated with urea | Japan: Nemuro-shi | LN851004 | LM652389 | LN851058 | LN851111 |
| A.caviariforme | Microascus caviariformis | CBS 536.87 (Ex-type) | Decaying meat | Belgium: Flemalle | LN851005 | LM652392 | LN851059 | LN851112 |
| Cephalotrichum asperulum | Doratomyces asperulus | CBS 127.22 | Seed | Netherlands: Wageningen | LN851006 | LN850959 | LN851060 | LN851113 |
| Doratomyces asperulus | CBS 582.71 (Ex-isotype) | Soil | Argentina: Buenos Aires | LN851007 | LN850960 | LN851061 | LN851114 | |
| Doratomyces sp. | UTHSCSA DI14-62; FMR 13443 | BAL | USA | LN851008 | LN850961 | LN851062 | LN851115 | |
| Doratomyces sp. | UTHSCSA DI14-65; FMR 13446 | BAL | USA | LN851009 | LN850962 | LN851063 | LN851116 | |
| C.brevistipitatum | Doratomyces purpureofuscus | CBS 157.57 (Ex-type) | Tuber | Netherlands: Wageningen | LN851031 | LN850984 | LN851084 | LN851138 |
| C.columnare | Doratomyces columnaris | CBS 159.66 (Ex-type) | Dung of hare | South Africa: Johannesburg | LN851010 | LN850963 | LN851064 | LN851117 |
| C.cylindricum | Trichurus terrophilus | CBS 448.51 | Timber | South Africa: Bekker | LN851011 | LN850964 | LN851065 | LN851118 |
| Trichurus cylindricus | UAMH 1348 (Ex-epitype) | Seed of sorghum | USA: Kansas | LN851012 | LN850965 | LN851066 | LN851119 | |
| C.dendrocephalum | Trichurus dendrocephalus | CBS 528.85 (Ex-isotype) | Cultivated soil | Iraq: Basrah | LN851013 | LN850966 | LN851067 | LN851120 |
| C.gorgonifer | Trichurus spiralis | CBS 131.08 | Unknown | Unknown | LN851021 | LN850974 | – | LN851128 |
| Trichurus terrophilus | CBS 368.53 | Treated wood | South Africa | LN851023 | LN850976 | LN851076 | LN851130 | |
| Trichurus spiralis | CBS 635.78 (Ex-epitype) | Hair | Netherlands | LN851024 | LN850977 | LN851077 | LN851131 | |
| Trichurus spiralis | UAMH 3585 | Mushroom compost | Canada: Alberta | LN851025 | LN850978 | LN851078 | LN851132 | |
| Doratomyces sp. | UTHSCSA DI14-63; FMR 13444 | BAL | USA | LN851026 | LN850979 | LN851079 | LN851133 | |
| Doratomyces microsporus | UTHSCSA DI14-64; FMR 13445 | BAL | USA | LN851027 | LN850980 | LN851080 | LN851134 | |
| Doratomyces sp. | UTHSCSA DI14-69; FMR 13450 | BAL | USA | LN851028 | LN850981 | LN851081 | LN851135 | |
| Doratomyces purpureofuscus | UTHSCSA DI14-71; FMR 13452 | Maxillary sinus fluid | USA | LN851029 | LN850982 | LN851082 | LN851136 | |
| Trichurus spiralis | UTHSCSA DI14-75; FMR 13456 | BAL | USA | LN851030 | LN850983 | LN851083 | LN851137 | |
| C.hinnuleum | Doratomyces stemonitis | CBS 289.66 (Ex-type) | Dung of deer | Australia: Tasmania | LN851032 | LN850985 | LN851085 | LN851139 |
| C.microsporum | Doratomyces purpureofuscus | CBS 523.63 (Ex-epitype) | Wheat field soil | Germany: Schleswig-Holstein | LN851014 | LN850967 | LN851068 | LN851121 |
| Cephalotrichum microsporum | UAMH 9365 | Indoor air | Canada: Alberta | LN851015 | LN850968 | LN851069 | LN851122 | |
| C.nanum | Doratomyces nanus | CBS 191.61 (Ex-epitype) | Dung of deer | England: Surrey | LN851016 | LN850969 | LN851070 | LN851123 |
| Cephalotrichum nanum | UAMH 9126 | Dung of bison | Canada: Alberta | LN851017 | LN850970 | LN851071 | LN851124 | |
| C.purpureofuscum | Cephalotrichum purpureofuscum | UAMH 9209 | Indoor air | Canada: British Columbia | LN851018 | LN850971 | LN851072 | LN851125 |
| C.stemonitis | Doratomyces stemonitis | CBS 103.19 (Ex-neotype) | Seed | Netherlands: Wageningen | LN850952 | LN850951 | LN850953 | LN850954 |
| Doratomyces stemonitis | CBS 180.35 | Unknown | Unknown | LN851019 | LN850972 | LN851073 | LN851126 | |
| Cephalotrichum stemonitis | UAMH 1532 | Unknown | Unknown | LN851020 | LN850973 | LN851074 | LN851127 | |
| C.verrucisporum | Doratomyces asperulus | CBS 187.78 | Sand dune soil | Netherlands: Katijk | LN851033 | LN850986 | LN851086 | LN851140 |
| Fairmania singularis | Microascus singularis | CBS 249.64 | Unknown | Canada: Toronto | LN851034 | LN850987 | LN851087 | LN851141 |
| Microascus singularis | CBS 414.64 | Laboratory contaminant | Japan: Tokyo | LN851035 | LM652442 | LN851088 | LN851142 | |
| Microascus singularis | CBS 505.66 (Ex-epitype) | Barrel bottom | USA: Maine | LN851036 | LN850988 | LN851089 | LN851143 | |
| Gamsia aggregata | Wardomyces aggregatus | CBS 251.69 (Ex-isotype) | Dung of carnivore | USA | LN851037 | LM652378 | LN851090 | LN851144 |
| G.columbina | Wardomyces columbinus | CBS 230.82 | Sandy soil | Netherlands: Wageningen | LN851038 | LN850989 | LN851091 | LN851145 |
| Wardomyces columbinus | CBS 233.66 (Ex-epitype) | Sandy soil | Germany: Giessen | LN851039 | LN850990 | LN851092 | LN851146 | |
| Wardomyces dimerus | CBS 235.66 (Ex-type) | Wheat field soil | Germany: Schleswig-Holstein | LN851040 | LN850991 | LN851093 | LN851147 | |
| Wardomyces simplex | CBS 546.69 (Ex-type) | Milled Oryza sativa | Japan | LN851041 | LM652379 | LN851094 | LN851148 | |
| Scopulariopsis brevicaulis | Microascus brevicaulis | MUCL 40726 (Ex-type) | Indoor air | Canada: Alberta | LN851042 | LM652465 | HG380363 | LM652672 |
| Microascus longirostris | Microascus longirostris | CBS 196.61 (Ex-neotype) | Wasp's nest | USA: Maine | LN851043 | LM652421 | LM652566 | LM652634 |
| Wardomyces anomalus | Wardomyces anomalus | CBS 299.61 (Ex-epitype) | Air cell of egg | Canada: Ontario | LN851044 | LN850992 | LN851095 | LN851149 |
| W.giganteus | Microascus giganteus | CBS 746.69 (Ex-type) | Insect frass in dead log | Canada: Ontario | LN851045 | LM652411 | LN851096 | LN851150 |
| W.humicola | Wardomyces humicola | CBS 369.62 (Ex-isotype) | Soil in tropical greenhouse | Canada: Ontario | LN851046 | LN850993 | LN851097 | LN851151 |
| W.inflatus | Wardomyces hughesii | CBS 216.61 (Ex-isotype) | Wood, Acer sp. | Canada: Québec | LN851047 | LM652496 | LN851098 | LN851152 |
| Wardomyces inflatus | CBS 367.62 (Ex-neotype) | Greenhouse soil | Belgium: Heverlee | LN851048 | LN850994 | LN851099 | LN851153 | |
| W.moseri4 | Wardomyces moseri | CBS 164.80 (Ex-isotype) | Dead petiole | Colombia: Dep. Meta | LN851049 | LN850995 | LN851100 | LN851154 |
| W.ovalis | Wardomyces ovalis | CBS 234.66 (Ex-type) | Wheat field soil | Germany: Schleswig-Holstein | LN851050 | LN850996 | LN851101 | LN851155 |
| W.pulvinatus | Wardomyces papillatus | CBS 112.65 (Ex-type) | Salt-marsh | England: Cheshire | LN851051 | LN850997 | LN851102 | LN851156 |
| Wardomycopsis humicola | Scopulariopsis humicola | CBS 487.66 (Ex-isotype) | Soil | Canada: Ontario | LM652554 | LM652497 | LN851103 | LN851157 |
| Wardomycopsis humicola | FMR 3993 | Sediment of Ter river | Spain: Girona | LN851052 | LN850998 | LN851104 | LN851158 | |
| Wardomycopsis sp. | FMR 13592 | Soil | Spain: Reus | LN851053 | LN850999 | LN851105 | LN851159 | |
| Ws.inopinata | Wardomycopsis inopinata | FMR 10305 | Soil | Myanmar | LN851054 | LM652498 | LN851106 | LN851160 |
| Wardomycopsis inopinata | FMR 10306 | Soil | Myanmar | LN850956 | LN850955 | LN850957 | LN850958 | |
| Ws.litoralis | Wardomycopsis litoralis | CBS 119740 (Ex-type) | Beach soil | Spain: Castellón | LN851055 | LN851000 | LN851107 | LN851161 |
CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; FMR: Facultat de Medicina i Ciències de la Salut, Reus, Spain; IHEM: Biomedical Fungi and Yeasts Collection, Scientific Institute of Public Health, Belgium; MUCL: Université Catholique de Louvain, Louvain-la-Neuve, Belgium; UAMH: UAMH Centre for Global Microfungal Biodiversity, University of Toronto, Canada; UTHSCSA: Fungus Testing Laboratory, Department of Pathology, University of Texas Health Science Center, San Antonio, USA.
BAL: bronchoalveolar lavage fluid.
ITS: Internal transcribed spacer regions of the rDNA and 5.8S region; LSU: partial large subunit of the rDNA; EF-1α: partial translation elongation factor gene; TUB: partial beta-tubulin gene.
Excluded or doubtful species name. Sequences newly generated in this study are indicated in bold.
Phenotypic characters
The isolates were grown on oatmeal agar (OA; 30 g of filtered oat flakes, 20 g of agar, 1 L of distilled water), potato-carrot agar (PCA; 20 g each of filtered potatoes and carrots, 20 g of agar, 1 L of distilled water) and potato dextrose agar (PDA, Pronadisa, Spain), incubated in the dark at different temperatures (5–40 °C at intervals of 5 °C) and examined at 7, 14 and 28 d to determine colony growth rates. Cultural and micro-morphological characteristics were recorded after 14 d of incubation at 25 °C on OA. Colour notations were from Kornerup & Wanscher (1978). Measurements and descriptions of microscopic structures were made using an Olympus CH2 light microscope (Olympus Corporation, Tokyo, Japan). Photographs were made using a Zeiss Axio Imager M1 light microscope (Zeiss, Oberkochen, Germany) with a mounted DeltaPix Infinity X digital camera using Nomarski differential interference contrast and phase contrast optics or using an Olympus SZ61 stereomicroscope with a mounted Olympus SC30 digital camera (Olympus, Tokyo, Japan). Cardinal temperatures were determined using PDA plates incubated at temperatures ranging from 5 to 40 °C at 5 °C intervals, including 37 °C.
DNA extraction, sequencing and PCR amplification
Total genomic DNA was extracted from fresh mycelia using FastPrep (MP Biomedicals, Santa Ana, California, USA) following the manufacturer's protocol. DNA was quantified using Nanodrop 3000 (Thermo Scientific, Madrid, Spain).
Four nuclear DNA regions were amplified by PCR following previously described conditions (Sandoval-Denis et al. 2016). The ITS region (ITS) of nuclear rDNA (nrDNA), spanning the ITS1, 5.8S and ITS2 regions, was amplified using the primer pair ITS5/ITS4 (White et al. 1990). LSU nrDNA region, spanning the variable domains D1–D3, was amplified using the primer pair LR5/LR0R (Vilgalys and Hester, 1990, Vilgalys and Sun, 1994). In addition, two protein coding genes were also used. Partial fragments of the translation elongation factor 1α (EF-1α) and β-tubulin (TUB) genes were amplified using the primer pairs 983F/2218R (Rehner & Buckley 2005) and BT2a/BT2b (Glass & Donaldson 1995), respectively. Sequencing was made in both directions with the same primers used for amplification at Macrogen Europe (Macrogen Inc. Amsterdam, The Netherlands). Consensus sequences were obtained using SeqMan v. 7.0.0 (DNASTAR Lasergene, Madison, WI, USA). Sequences newly generated in this study and their GenBank accession numbers are shown in Table 1.
Sequence alignment and phylogenetic analysis
Alignments of individual genes were created in MEGA v. 6 (Tamura et al. 2013), using the ClustalW function and refined in the same platform manually or using Muscle (Edgar 2004). The best-fit models of evolution for the four genes tested (GTR+I+G for LSU, ITS and EF-1α; and HKY+I+G for TUB) were selected following the Akaike criterion (AIC) (Posada & Buckley 2004) implemented in MrModelTest v. 2.3 (Nylander 2004). Microascus longirostris (CBS 196.61) and Scopulariopsis brevicaulis (MUCL 40726) were used as outgroups. Maximum likelihood (ML) analyses were performed using MEGA v. 6 with Nearest-Neighbour-Interchange as a heuristic method. Gaps were treated as partial deletions with a 95 % site coverage cut-off. Robustness of the branches was estimated using a bootstrap analysis of 1 000 replicates (Felsenstein 1985). Bootstrap values ≥70 % were considered significant. Bayesian (BI) analyses were conducted on MrBayes v. 3.2 (Huelsenbeck & Ronquist 2001) and involved two parallel runs of four incrementally heated Markov Chains starting from a random tree topology. The analyses lasted for 5 M generations with a sampling frequency of every 100 generations. The 50 % majority rule consensus trees and posterior probabilities (pp) were calculated after discarding 25 % of the initial trees for burn-in. Posterior probability values equal or above 0.95 were considered significant. The resulting trees were plotted using FigTree v. 1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/). Individual datasets of ITS, LSU, EF-1α and TUB were assessed for potential incongruence before being concatenated into a combined dataset. If conflict between clades with significant ML and BI support was observed, the individual phylogenies were considered to be incongruent (Mason-Gamer and Kellogg, 1996, Wiens, 1998). However, since no incongruences were found, all genes were combined for the final phylogenetic analyses. The alignments originated in this study have been deposited in TreeBASE (http://www.treebase.org) and taxonomic novelties in MycoBank (Crous et al. 2004).
Results
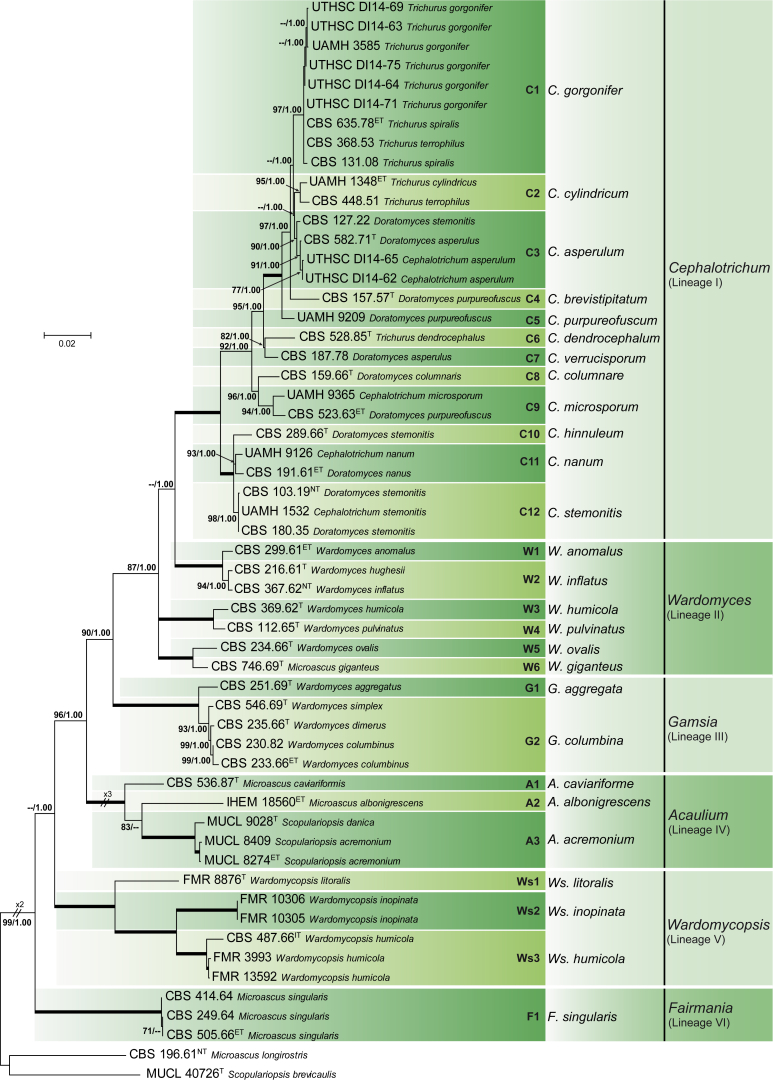
The final dataset comprised 2 595 characters (819 characters for LSU, 504 for ITS, 772 for EF-1α and 500 for TUB) and included 584 parsimony-informative positions (74 for LSU, 119 for ITS, 162 for EF-1α and 229 for TUB) from 56 isolates. The resulting ML phylogenetic tree (Fig. 1) resolved 27 well-supported terminal clades distributed in six main lineages (I–VI), which corresponded to six different genera, i.e. Acaulium, Cephalotrichum, Fairmania, Gamsia, Wardomyces and Wardomycopsis.
Fig. 1.
Maximum likelihood (ML) tree obtained from the combined LSU, ITS, EF-1α and TUB sequences of 57 representative taxa of the Microascaceae. Numbers on the branches are ML bootstrap values (bs) above 70 %, followed by Bayesian posterior probabilities (pp) above 0.95. Fully supported branches (100 % bs/1.0 pp) are indicated in bold. Branch lengths are proportional to distance. The tree is rooted to Microascus longirostris (CBS 196.61) and Scopulariopsis brevicaulis (MUCL 40726). T, Ex-type; ET, Ex-epitype; NT, Ex-neotype; A. Acaulium; C. Cephalotrichum; F. Fairmania; G. Gamsia; W, Wardomyces; Ws, Wardomycopsis.
Lineage I corresponded to the genus Cephalotrichum which encompassed 12 terminal clades (C1–C12); nine of which included an ex-type, ex-neotype or ex-epitype strain of a known species. Two clades (C4 and C10) corresponded to new species described here as C. brevistipitatum and C. hinnuleum, and clades C5 and C7 included a single strain which were identified respectively as C. purpureofuscum and C. verrucisporum (see notes on those species). The species phylogenetically attributed to Cephalotrichum are characterised by forming synnematous conidiophores with or without sterile distal setae and annellidic conidiogenesis producing smooth or rough conidia arranged in basipetal chains.
Lineage II showed considerable phylogenetic diversity among species of Wardomyces that grouped into six clades distributed into three paraphyletic sublineages. Each of these six clades (W1–W6) included an ex-type or authentic strain for the species. Members of this lineage are characterised by the formation of usually branched conidiophores with polyblastic conidiogenous cells producing solitary, dark 1–2-celled conidia bearing a germ slit. An exception is W. ovalis (clade W5) that presented secondary annellidic, hyaline, 1-celled conidia arranged in chains. Given the lack of clear morphological differences, we interpreted these six clades as belonging to Wardomyces sensu lato.
Lineage III corresponded to the genus Gamsia and comprised two terminal clades (G1 and G2), which represented two known species. Members of this lineage are characterised by usually unbranched conidiophores bearing polyblastic conidiogenous cells, and 1-celled, dark, solitary conidia provided with germ slits, and secondary, 1–2-celled, hyaline annelloconidia in long chains.
Lineage IV comprised three terminal clades (A1–A3), each of which included an ex-type or a reference strain of a known species. One of them, Microascus albonigrescens, is the type species of the obscure genus Acaulium, which is reintroduced here. Acaulium is characterised by annellidic conidiogenesis, guttulate conidia and mycelium forming abundant hyphal fascicles.
Lineage V included three clades (Ws1–Ws3) corresponding to the genus Wardomycopsis and the currently accepted species Ws. litoralis, Ws. humicola and Ws. inopinata. Although the ex-type strain of Ws. inopinata, the type species of the genus, was unavailable for study, specimens in clade Ws2 were considered representative of that species in that they match morphologically with the protologue of Ws. inopinata (Udagawa & Furuya 1978). Members of Wardomycopsis are characterised by hyaline annellidic conidiogenous cells producing conidia in short chains; the conidia are darkly pigmented and have a single longitudinal germ slit.
Lineage VI corresponded to the obscure genus Fairmania, which is reintroduced here. This lineage included a single terminal clade (F1), which comprises three strains of F. singularis characterised by dark, 1-celled conidia with 1–5 longitudinal paler bands.
Taxonomy
Acaulium Sopp, Skr. VidenskSelsk. Christiania, Kl. I, Math.-Natur. 11: 42. 1912.
Colonies expanding, often membranous at first, becoming velvety, lanose or funiculose, flat, white to pale grey. Hyphae hyaline, thin- and smooth-walled, often forming fascicles. Conidiophores mononematous, rarely synnematous, branched or unbranched, hyaline. Conidiogenous cells annellidic, cylindrical, smooth-walled. Conidia obovoid to cylindrical, hyaline or subhyaline, smooth- and thick-walled, truncate at the base. Ascomata superficial or immersed, scattered, perithecial and papillate or cleistothecial, black, with scattered setae. Asci evanescent, 8-spored, subglobose to globose. Ascospores 1-celled, lunate, pale orange to red-brown, smooth-walled, with a single apical germ pore.
Type species: Acaulium albonigrescens Sopp.
Notes: Species currently included in Acaulium were recently excluded from Microascus and Scopulariopsis on the basis of DNA phylogenetic analysis (Sandoval-Denis et al. 2016). Although the morphological distinction among the three genera is difficult, Acaulium is characterised by the formation of pale colonies with dense hyphal fascicles and the presence of abundant oil drops in the mycelium, conidia and ascospores, showing a guttulate appearance. In addition, species of Acaulium are able to grow at low temperature, sporulating abundantly at 15 °C, whereas in Microascus and Scopulariopsis, sporulation is markedly reduced at temperatures below 25 °C.
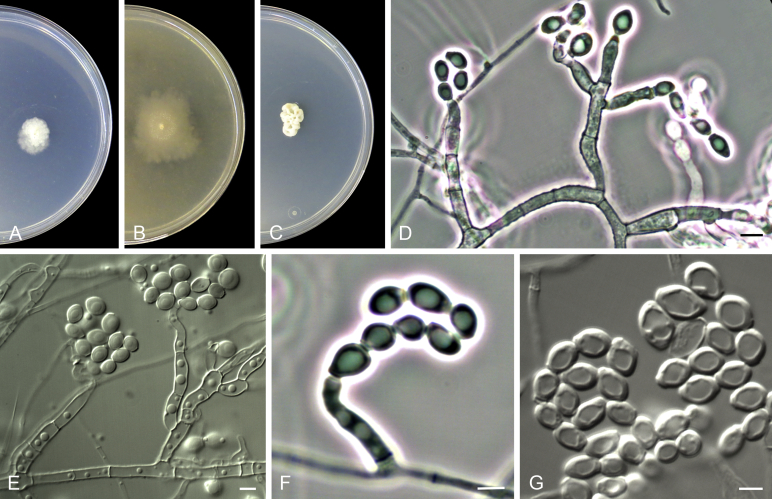
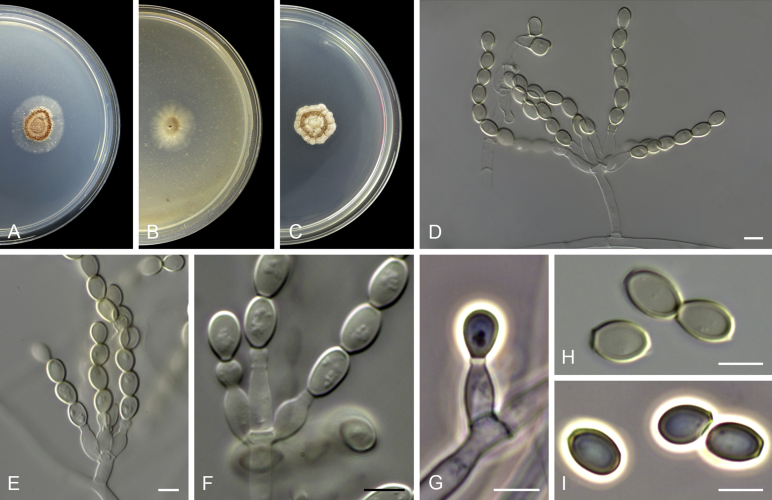
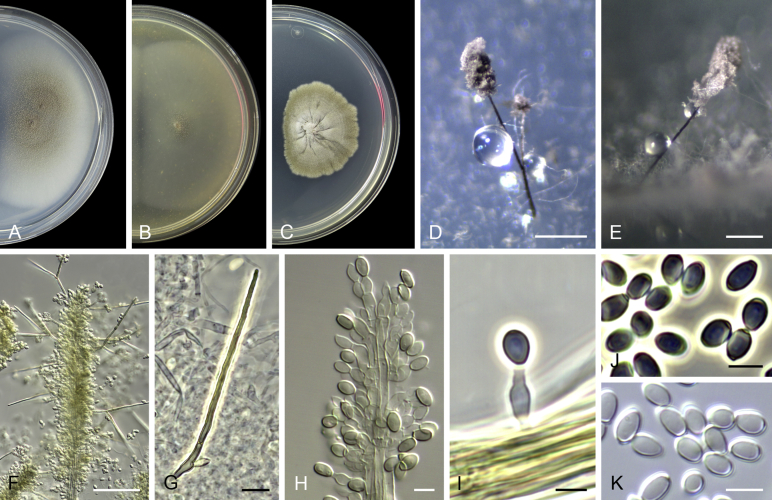
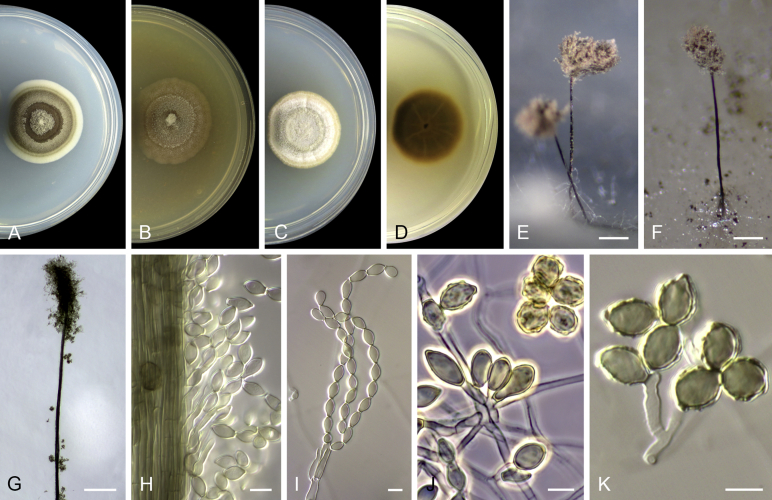
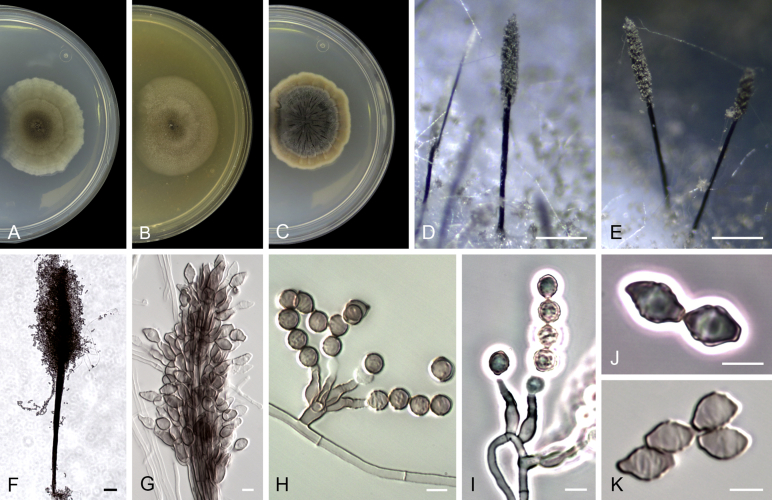
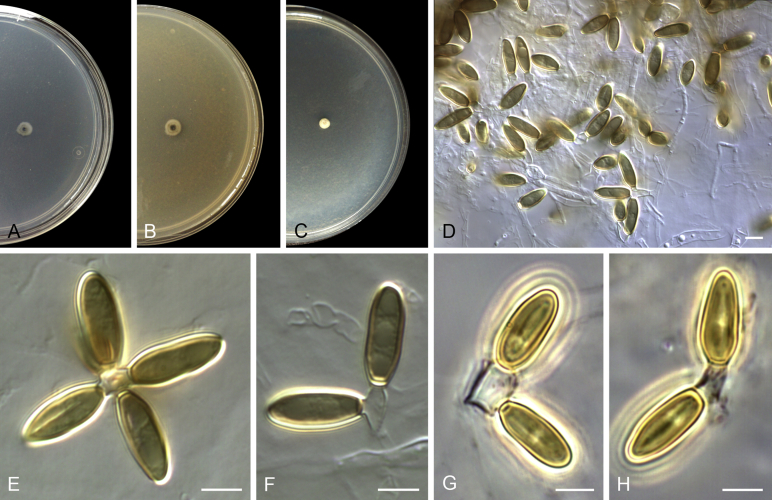
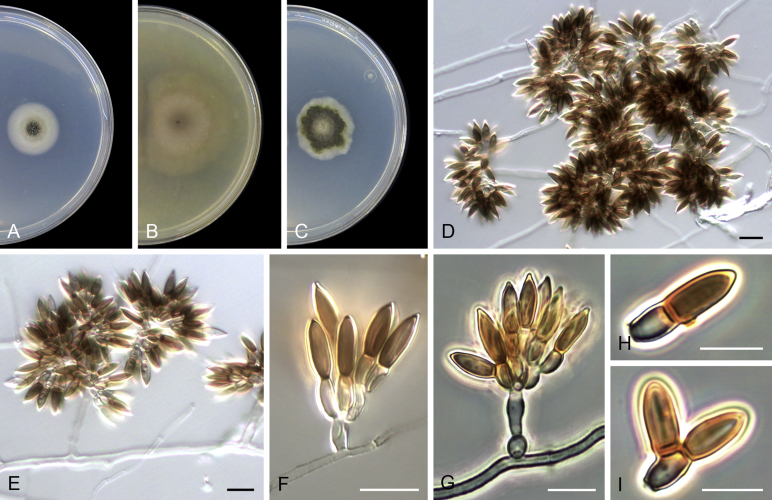
Acaulium acremonium (Delacr.) Sandoval-Denis, Guarro & Gené, comb. nov. MycoBank MB814571. Fig. 2.
Fig. 2.
Acaulium acremonium (ex-epitype MUCL 8274). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–G. Conidiophores, annellides and conidia. Scale bars: D–G = 10 μm.
Basionym: Monilia acremonium Delacr., Bull. Soc. Mycol. France 13: 114. 1897.
Synonyms: Scopulariopsis acremonium (Delacr.) Vuill. Bull. Soc. Mycol. France. 27: 148. 1911.
Scopulariopsis communis Bainier, Bull. Soc. Mycol. France. 23: 125. 1907.
Penicillium brevicaule Sacc. var. glabrum Thom, Bull. Off. Exp. Sta. U. S. D. A. 118: 48. 1910.
Scopulariopsis brevicaulis (Sacc.) Bainier var. glabra (Thom) Thom in The Penicillia: 250. 1930.
Oospora glabra Hanzawa, J. Coll. Agric. Tohoku Imp. Univ. 4: 1912.
Penicillium scopulariopsis Sacc., Syll. Fung. 22: 1275. 1913.
Scopulariopsis candelabrum Loubière, Rech. struct. Mucor., (Thesis), Paris: 63. 1924.
Scopulariopsis danica F.H. Beyma, Zentralbl. Bakteriol. Parasitenk., Abt. 2. 99: 390. 1939.
Scopulariopsis communis Bainier var. lunzinensis S zilvinyi., Zentralbl. Bakteriol. Parasitenk., Abt. 2. 103: 173. 1941.
Material examined: Lectotype designated here: T. XIII, plate IX in Delacroix EG. 1897. Quelques espèces nouvelles. Bulletin de la Société Mycologique de France 13: 114–127, MBT-372234. Denmark, from skin of a horse infected with Trichophyton sp., 1938, C. Werdelin (ex-type culture of Scopulariopsis danica MUCL 9028). Epitype designated here: Germany, Schleswig-Holstein, Kiel-Kitzeberg, from wheat field soil, 1963, W. Gams, MBT-202769 (culture ex-epitype MUCL 8274 = CBS 104.65); Schleswig-Holstein, Kiel-Kitzeberg, from soil, 1965, W. Gams (MUCL 8409).
Description and illustrations: Morton & Smith (1963).
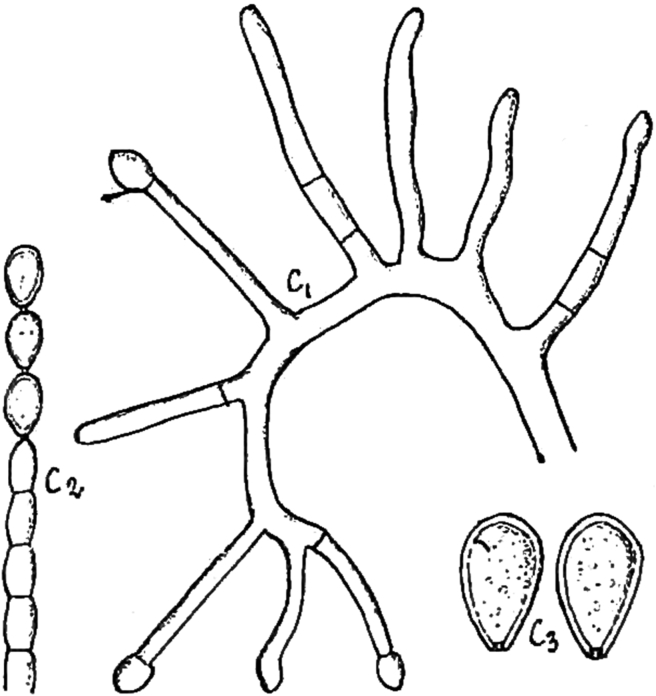
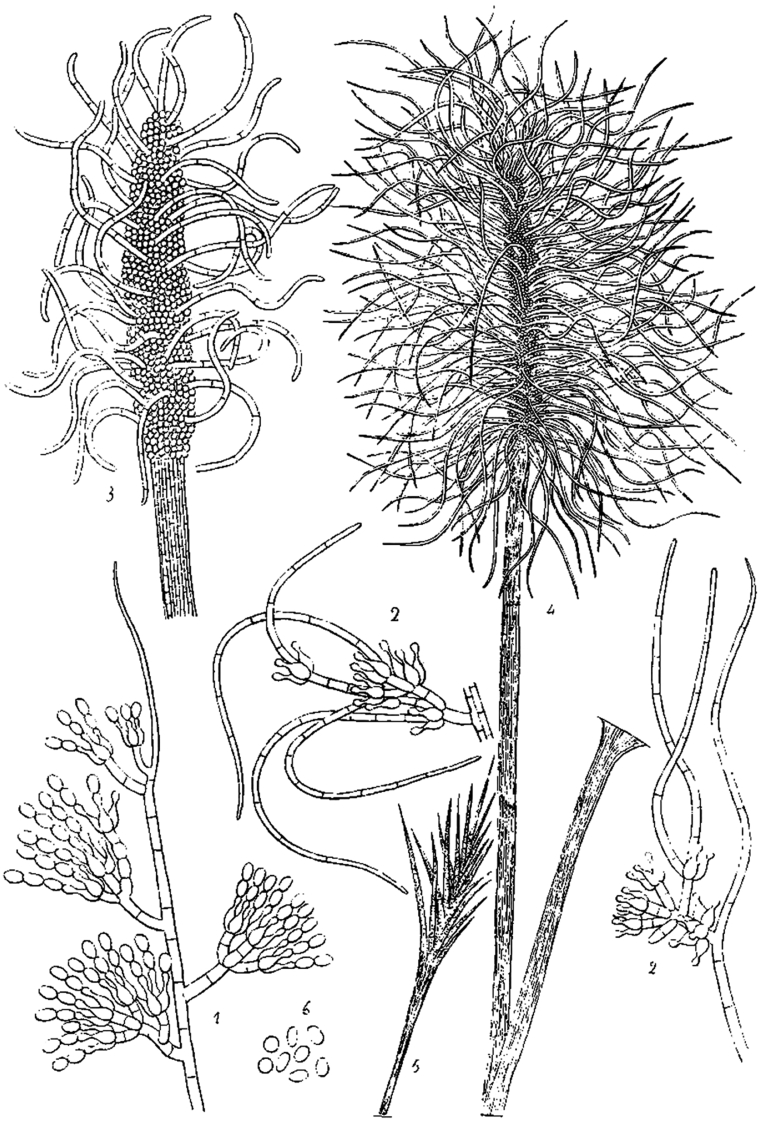
Notes: Delacroix (1897) described Monilia acremonium from rotten paper found in garbage, but no type material is known. The ex-type strain of Scopulariopsis danica, studied here and considered a heterotypic synonym of S. acremonium, can be still recognised by its morphological features, although, as previously documented, this culture is in bad condition with poor sporulation (Morton & Smith 1963). Holotype material is unavailable for this species. However, the protologue contains an illustration that serves as lectotype, which is designated and reproduced here (Fig. 3). Given that only a limited number of strains of A. acremonium exist and in order to fix the use of the name, we have selected the strain MUCL 8274 as epitype. Although it has conidia slightly smaller (5–12 × 3–6 μm) than those described in the protologue of M. acremonium, their size ranges are close to that given by Morton & Smith (1963) for S. acremonium. A number of additional North American isolates from soil and clinical sources examined by one of us (Abbott 2000) are consistent with S. acremonium as circumscribed by Morton & Smith (1963) and support the epitypification proposed here.
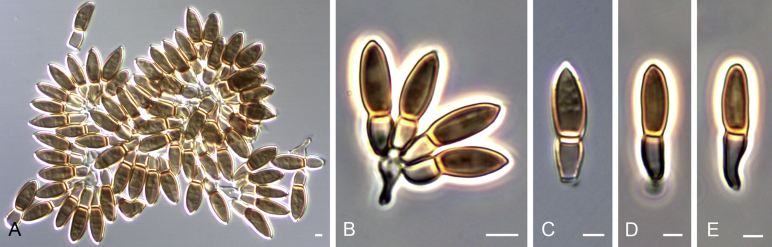
Fig. 3.
Reproduction of the original drawings by Delacroix (1897) illustrating Monilia acremonium (original numbers are maintained to indicate the different structures). C1. Conidiogenous cells and conidia. C2. Conidial chain. C3. Conidia.
This species has been reported as causing skin and nail infections in humans (de Hoog et al. 2011); however, its identification from cases of proven clinical infection has not been confirmed by molecular methods (Sandoval-Denis et al. 2013). Acaulium acremonium is only known by its asexual morph characterised by large, often pointed ovate conidia produced on long cylindrical and somewhat curved annellides borne on branched or unbranched conidiophores. The closely related species A. albonigrescens produces smaller (5.5–8 × 2–3.5 μm), clavate to cylindrical conidia with rounded apices, formed on straight cylindrical annellides and the species further differs by showing a sexual morph.
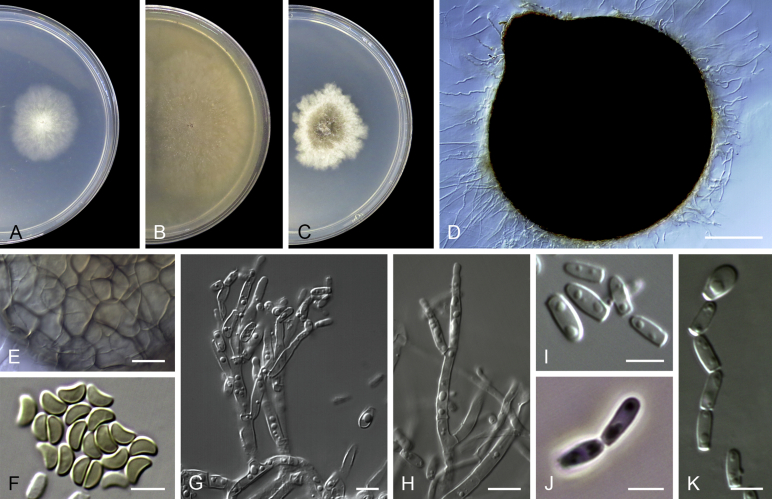
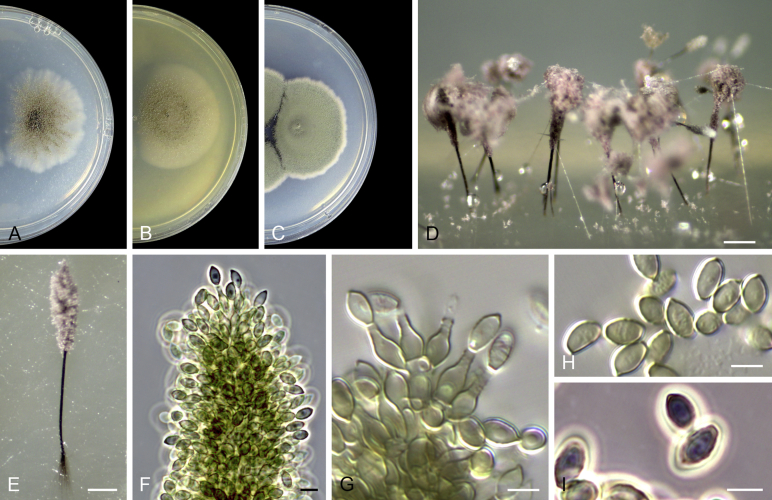
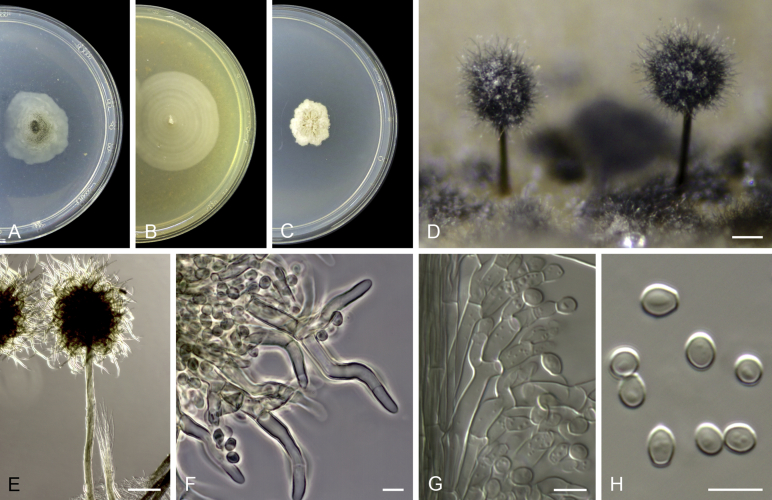
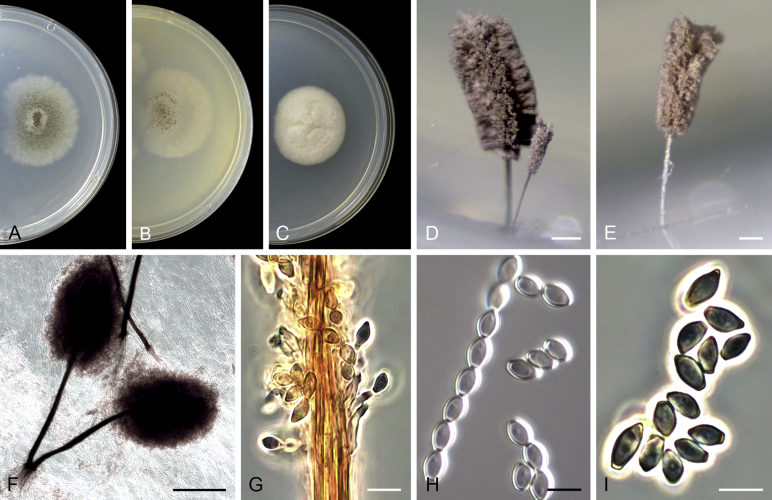
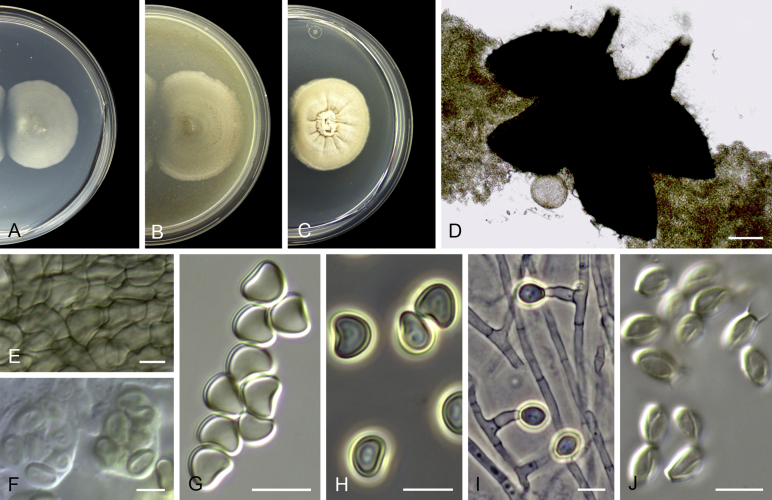
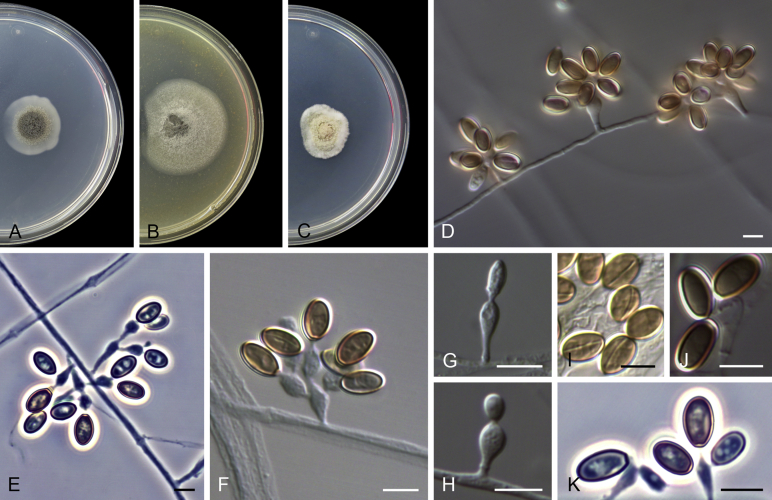
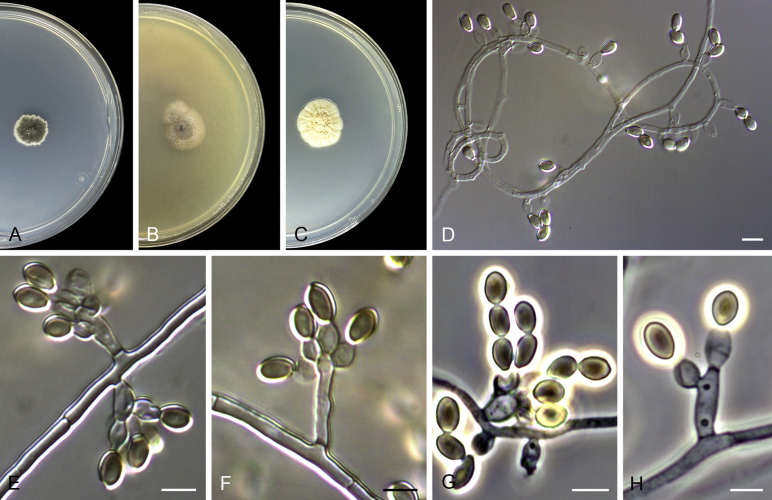
Acaulium albonigrescens Sopp, Skr. Vidensk.-Selsk. Christiana Math.-Nat. Kl. 11: 70. 1912. Fig. 4.
Fig. 4.
Acaulium albonigrescens (ex-epitype IHEM 18560). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D. Ascoma. E. Ascomatal peridium. F. Ascospores. G–H. Conidiophores and conidiogenous cells. I–K. Conidia. Scale bars: D = 100 μm; E–K = 5 μm.
Synonyms: Microascus albonigrescens (Sopp) Curzi, Boll. Staz. Patol. Veg. Roma 11: 60. 1931.
Penicillium albonigrescens (Sopp) Sacc. [as ‘alba-nigrescens’], Syll. Fung. 25: 670. 1931.
Material examined: Lectotype designated here: plates VI–VII in Sopp OJ. 1912. Monographie der Pilzgruppe Penicillium mit besonderer Berücksichtigung der in Norwegen gefunden Arten. Videnskaps Selskapets Skrifter. 1. Mat-Naturv Klasse 11: 1–207, MBT-372235; Epitype designated here: Japan, Nemuro-shi, Hokkaido, from litter treated with urea, 1967, S. Udagawa, MBT-202737 (CBS H-22334, culture ex-epitype IHEM 18560 = CBS 109.69).
Description and illustrations: Barron et al. (1961).
Notes: Although no authentic material exists and Morton & Smith (1963) list the species as “unidentifiable”, the modern concept of this taxon (as Microascus albonigrescens) was based in herbarium material and a living isolate described by Barron et al. (1961). The protologue of the species includes numerous drawings and aquarels which are thus proposed here as lectotype (Fig. 5). The isolate studied and proposed as epitype (IHEM 18560) conforms with the morphological characteristics of descriptions of M. albonigrescens by Barron et al., 1961, Udagawa and Awao, 1969, Arx et al., 1988, and Lumley et al. (2000). Acaulium albonigrescens forms white colonies, guttulate, cylindrical to clavate, hyaline conidia (5.5–8 × 2–3.5 μm), and has a sexual morph characterised by lunate ascospores with rounded ends. Acaulium caviariforme, the other species of the genus producing a sexual morph, has darker colonies, shorter, obovoid to ellipsoidal, brown conidia (5–7 × 3–5 μm) and fusiform ascospores. Acaulium albonigrescens is a well-circumscribed species described from soil, dung and wood in northern areas (Scandinavia, northern North America and Japan).
Fig. 5.
Reproduction of the original drawings by Sopp (1912) illustrating Acaulium albonigrescens (original numbers are maintained to indicate the different structures). A. Asexual morph: 40. Germinating conidia. 41–44. Conidiophores, conidiogenous cells and conidia. 45. Synnematal conidiophores. 46–51. Diverse phases of perithecial development, the first stages are seen in 46a and b. B. Sexual morph: 52. Sections of perithecia showing the ostiole development. 53. Horizontal section of perithecia embedded in the mycelium. 54. Mature perithecium. 55. Cross section of an immature perithecium. 56. Cross section of a fully mature perithecium, completely emptied through the ostiole. 57. Cross section of an empty perithecial ostiole. 58. Cross section of a perithecial ostiole during the liberation of ascospores. 59–60. Free ascospore masses. 61. Asci. 62. Ascospores. 63–65. Macroscopic features.
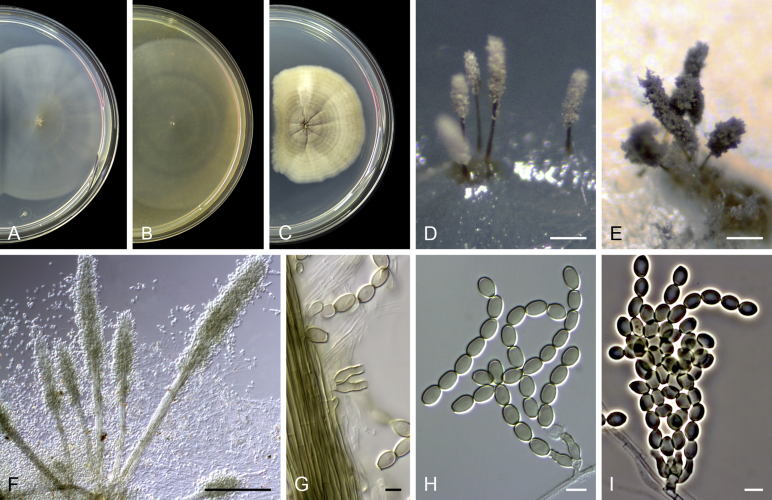
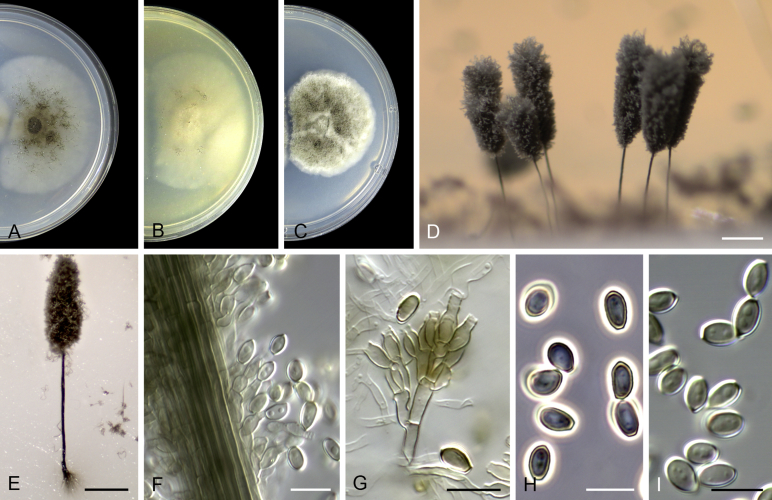
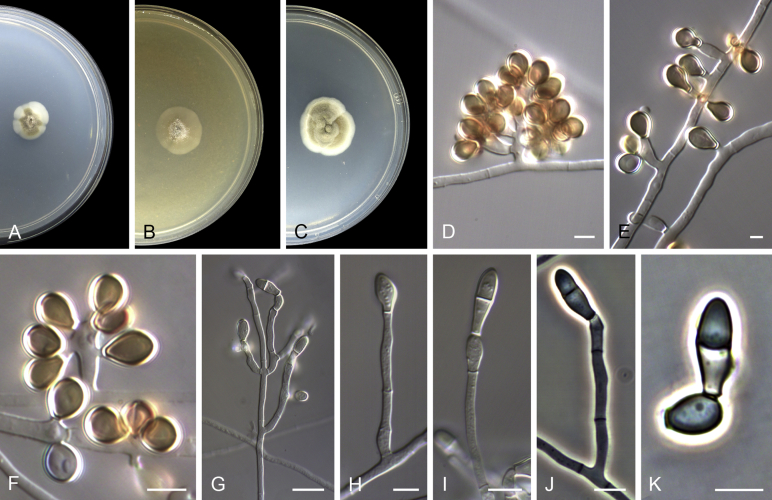
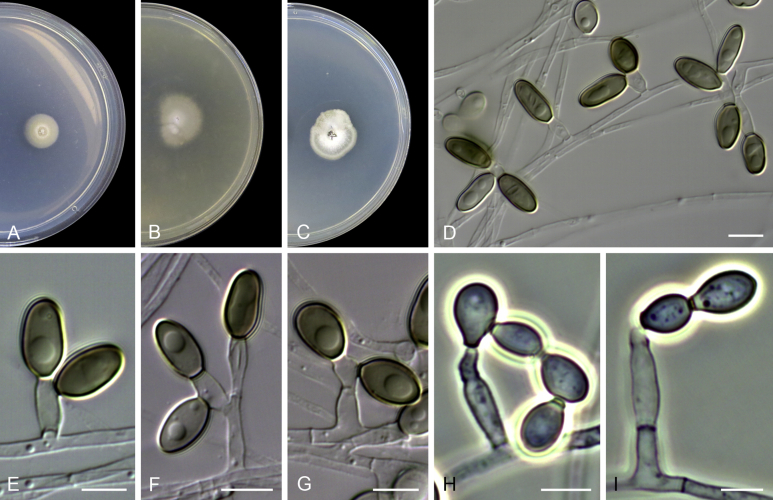
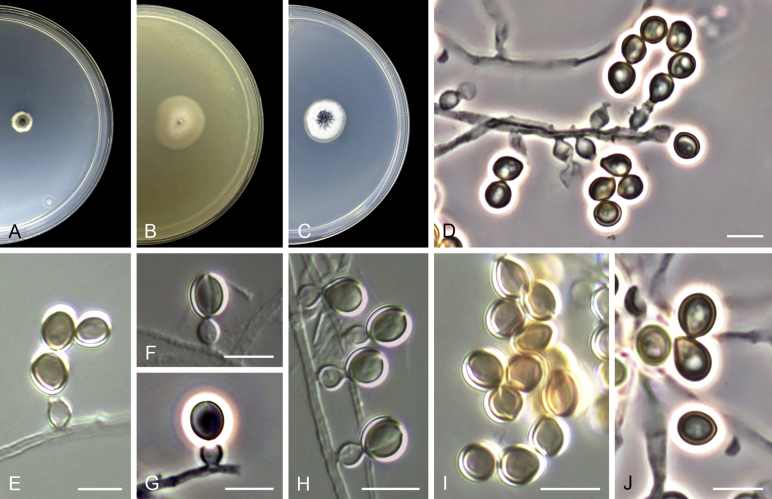
Acaulium caviariforme (Malloch & Hubart) Sandoval-Denis, Guarro & Gené comb. nov. MycoBank MB814573. Fig. 6.
Fig. 6.
Acaulium caviariforme (ex-type CBS 536.87). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–G. Conidiophores and conidiogenous cells. H–I. Conidia. Scale bars: D–I = 5 μm.
Basionym: Microascus caviariformis Malloch & Hubart, Canad. J. Bot. 65: 2384. 1987.
Material examined: Belgium, Prov. de Liège, Flemalle, Cave de Ramioul, from decaying meat, 1985, D.W. Malloch (Holotype TRTC 50940; culture ex-type CBS 536.87).
Description and illustrations: Malloch & Hubart (1987).
Notes: This species was originally placed in Microascus based on morphological features of the well developed sexual morph (Malloch & Hubart 1987). Phylogenetic analyses have demonstrated, however, that it grouped in a lineage separate from the above mentioned genus (Issakainen et al., 2003, Sandoval-Denis et al., 2016). In our phylogenetic analysis, the ex-type culture of A. caviariforme grouped with high statistical support with species of Acaulium. Acaulium caviariforme is morphologically similar to A. albonigrescens; both species produce sexual and asexual morphs in culture. However, A. caviariforme has fusiform, pale orange to copper-red ascospores, measuring 6–9 × 2–3 μm, and brown, obovoid to ellipsoidal conidia, 5–7 × 3–5 μm; ascospores of A. albonigrescens are smaller (3.5–5.5 × 2–3.5 μm), lunate and red-brown, and its conidia are clavate to cylindrical, hyaline and narrower (5.5–8 × 2–3.5 μm). Acaulium caviariforme appears to occupy a unique niche, having been isolated from meat in caves in Europe and North America.
Cephalotrichum Link, Mag. Ges. Naturf. Freunde Berlin 3: 20. 1809.
Synonyms: Doratomyces Corda, Sturm, Deutschl. Fl., Abt. 3 (Pilze Deutschl.) 2: 65. 1829.
Stelechotrichum Ritgen 1831 nom. inval. Publication not traced (Seifert et al. 2011).
Echinobotryum Corda, in Sturm, Deutschlands Flora, Abt. 3 (Pilze) 3: 51.1831.
Stysanus Corda, Icon. fung. (Prague) 1: 21. 1837.
Synpenicillium Costantin, Bull. Soc. Mycol. France. 4: 62. 1888.
Trichurus Clem. [& Shear], in Pound & Clements, Bot. Surv. Nebr. 4: 7. 1896.
Berkeleyna Kuntze, Revis. gen. pl. (Leipzig) 3: 447. 1898.
Stysanopsis Ferraris, Ann. Mycol. 7: 281. 1909.
Capnostysanus Speg., Physis 4: 295. 1918.
Colonies growing slowly to moderately fast, velvety, powdery, floccose, funiculose or fasciculate, flat, white, becoming pale to dark grey. Hyphae subhyaline to dark brown, rarely hyaline, thin- and smooth-walled. Conidiophores arising from the substratum or from the aerial mycelium, branched or unbranched, septate, smooth or finely ornamented, often aggregated in synnemata. Conidiogenous cells commonly penicillately arranged, annellidic, flask-shaped, subhyaline to dark brown and smooth-walled. Conidia basipetal, catenate, dry, 1-celled, obovoid, ellipsoidal, globose to subglobose broadly truncate at the base, hyaline or subhyaline, thin- or thick-walled, smooth or distinctly verrucose. Synnemata with a pale brown to black stipe and fertile at the upper portion forming a rounded to cylindrical sporulating head; sterile setae can be formed at the upper part of the synnemata, septate, long, cylindrical, branched or unbranched, straight or coiled. A second asexual state (referred as echinobotryum-like synasexual morph) can be present: conidiogenous cells polyblastic, borne solitary or on short penicillate conidiophores on the hyphae or on the synnemata; conidia grouped in clusters, oval to fusiform, often with a pointed apex, dark brown, verrucose and thick-walled.
Type species: Cephalotrichum stemonitis Link.
Notes: Cephalotrichum and other genera of the Microascaceae such as Microascus, Scopulariopsis and the recently proposed genus Fuscoannellis (Jagielski et al. 2016) have very similar conidiogenous apparatus, and asexual morphs of the genera could be hardly distinguished from each other, especially when isolates grow on rich culture media like PDA. However, although conidiophores in the three latter genera can arise from dense hyphal mycelial fascicles, they never form synnemata. Other genera of Microascaceae having synnematous conidiophores include Parascedosporium, Petriella and Scedosporium, but the conidia of these genera are produced in slimy masses and sexual morphs are produced in many species (Lackner et al. 2014). Cephalotrichum produces conidia in dry basipetal chains and sexual morphs are unknown.
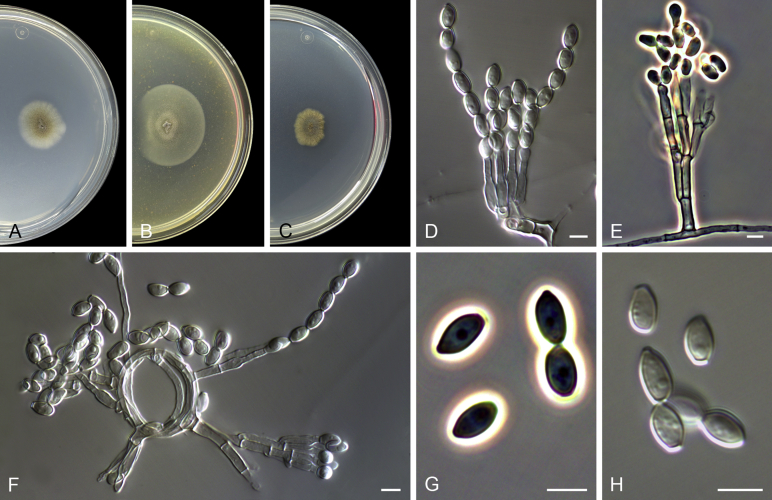
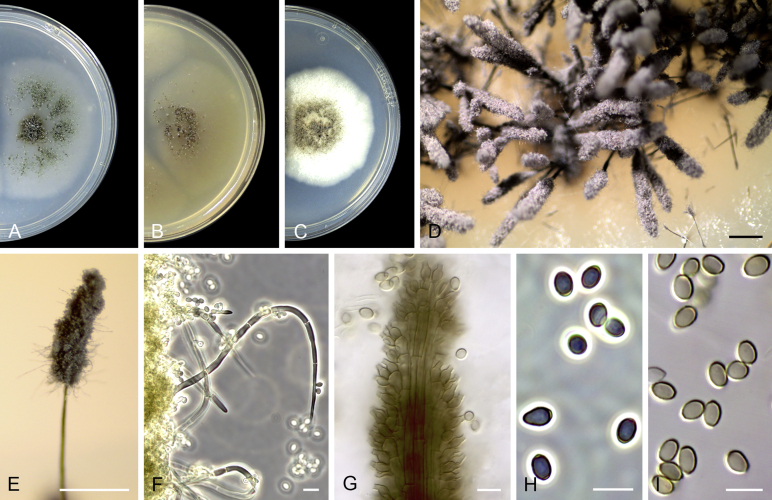
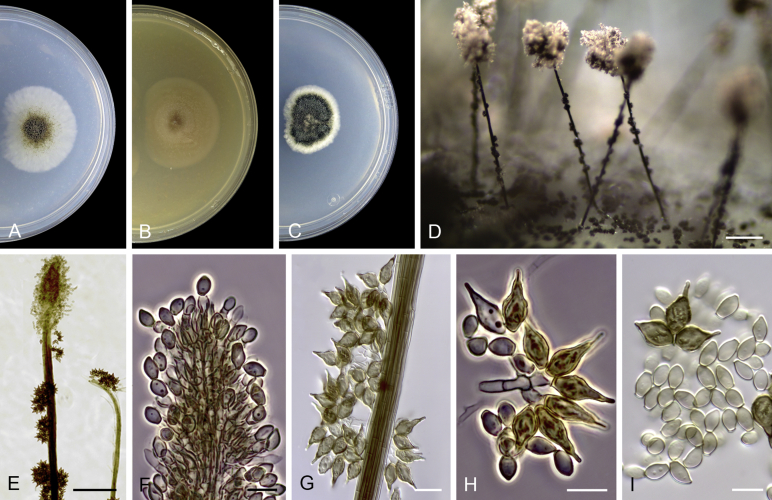
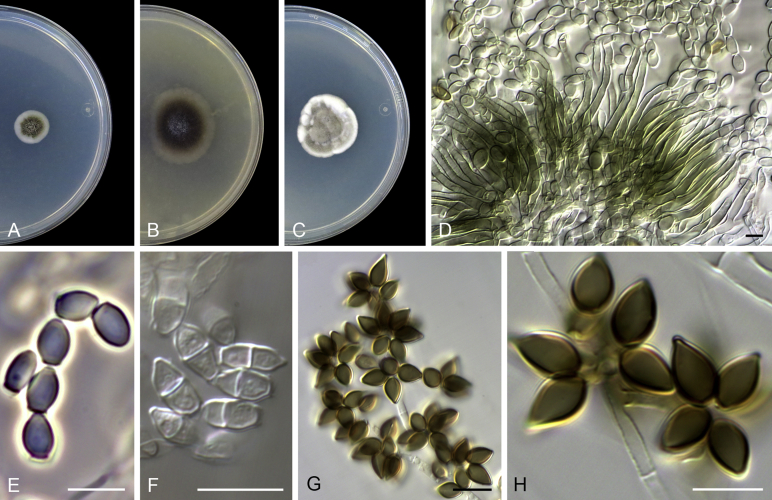
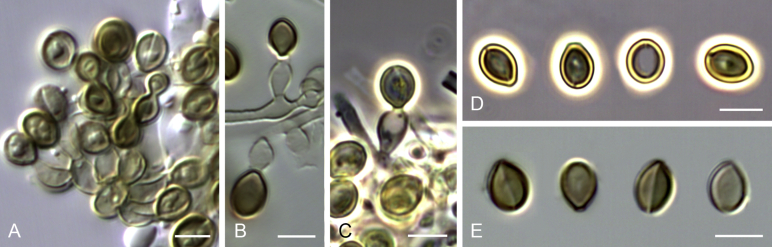
Cephalotrichum asperulum (J.E. Wright & S. Marchand) Sandoval-Denis, Guarro & Gené, comb. nov. MycoBank MB814577. Fig. 7
Fig. 7.
Cephalotrichum asperulum (ex-isotype CBS 582.71). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–E. Synnemata. F. Apical portion of a synnema. G. Conidiogenous cells. H–I. Conidia. Scale bars: D–E = 200 μm; F–I = 5 μm.
Basionym: Doratomyces asperulus J.E. Wright & S. Marchand, Bol. Soc. Argent. Bot. 14: 308. 1972.
Material examined: Argentina, Buenos Aires, Arroyo Las Viboras, humus-rich soil in low grassland, 1971, J.E. Wright (Holotype BAFC 2135; culture ex-isotype CBS 582.71). The Netherlands, Wageningen, from seed, 1922, C.M. Doyer (CBS 127.22 as Doratomyces stemonitis). USA, from bronchoalveolar lavage fluid, unknown date, D.A. Sutton (UTHSCSA DI14-62 = FMR 13443); from bronchoalveolar lavage fluid, unknown date, D.A. Sutton (UTHSCSA DI14-65 = FMR 13446).
Description and illustrations: Wright & Marchand (1972).
Notes: This clade includes isolates obtained from different environmental sources, as well as from clinical human specimens mainly from lower respiratory tract. However, its inability to grow at 37 °C (Table 2) suggests that its isolation from clinical samples could represent environmental contamination.
Table 2.
Relevant phenotypic features of members of Acaulium, Cephalotrichum, Fairmania, Gamsia, Wardomyces and Wardomycopsis.
| Species | Sexual morph | Asexual morph | Ascospore |
Synnemata size (μm) | Annelloconidia |
Solitary conidia |
Growth at (°C) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (μm) | Shape | Size (μm) | Shape, surface and colour | Size (μm) | Shape, surface and colour | 5 | 15 | 25 | 30 | 35 | 37 | ||||
| Acaulium | |||||||||||||||
| A. acremonium | − | + | n/a | n/a | n/a | 5–12 × 3–6 | Obovate, smooth and hyaline | n/a | n/a | + | + | + | + | + | − |
| A. albonigrescens | + | + | 3.5–5.5 × 2–3.5 | Lunate | n/a | 5.5–8 × 2–3.5 | Cylindrical to clavate, smooth and hyaline | n/a | n/a | + | + | + | + | − | − |
| A. caviariforme | + | + | 6–9 × 2–3 | Fusiform | n/a | 5–7 × 3–5 | Obovoid to ellipsoidal/smooth/brown | n/a | n/a | + | + | + | + | − | − |
| Cephalotrichum | |||||||||||||||
| C. asperulum | − | + | n/a | n/a | 120–1 000 | 5–8.5 × 3–4 | Oval to ellipsoidal, rough, pale brown | n/a | n/a | + | + | + | + | + | − |
| C. brevistipitatum | − | + | n/a | n/a | 300–500 | 6–7 × 3.5–4 | Ellipsoidal, smooth to finely roughened, pale brown | n/a | n/a | − | + | + | + | + | − |
| C. columnare | − | + | n/a | n/a | 50–500 | 5.5–7.5 × 2.5–4 | Oval to ellipsoidal, assymetrical, smooth, brown-black | n/a | n/a | + | + | + | + | + | + |
| C. cylindricum | − | + | n/a | n/a | 450–700 | 4.5–6 × 2.5–3.5 | Oval to ellipsoidal, smooth, pale green | n/a | n/a | + | + | + | + | + | + |
| C. dendrocephalum | − | + | n/a | n/a | 1 000–2 000 | 5–7 × 2.5–3.5 | Oval to ellipsoidal, smooth, grey-brown | n/a | n/a | + | + | + | + | + | − |
| C. gorgonifer | − | + | n/a | n/a | 500–1 000 | 4–8 × 2.5–4 | Oval to ellipsoidal, smooth, pale brown | n/a | n/a | + | + | + | + | + | + |
| C. hinnuleum | − | + | n/a | n/a | 800–1 600 | 6–7.5 × 2.5–4 | Subglobose to ellipsoidal, smooth, pale brown | 8.5–10 × 5.5–7 | Oval to navicular, warted, brown | + | + | + | + | − | − |
| C. microsporum | − | + | n/a | n/a | 500–1 000 | 3.5–5 × 2–3 | Oval to ellipsoidal, smooth, green-brown | n/a | n/a | − | + | + | + | − | − |
| C. nanum | − | + | n/a | n/a | 500–2 000 | 6–8.5 × 4.5–7.5 | Subspherical to oval, coarsely warted, grey-brown | n/a | n/a | + | + | + | + | − | − |
| C. purpureofuscum | − | + | n/a | n/a | 800–1 600 | 5–8 × 3–4.5 | Oval to ellipsoidal, smooth or slightly roughened, green-brown | n/a | n/a | + | + | + | + | + | − |
| C. stemonitis | − | + | n/a | n/a | 2 000–3 000 | 6–9 × 4–5 | Ellipsoidal to cylindrical, smooth, pale green-brown | 8–19 × 6–7.5 | Fusoid, coarsely warted, dark-brown | + | + | + | + | − | − |
| C. verrucisporum | − | + | n/a | n/a | 1 000–3 000 | 6–9 × 3–5.5 | Globose to oval, rough, dark brown | n/a | n/a | + | + | + | + | − | − |
| Fairmania | |||||||||||||||
| F. singularis | + | + | 4.5–7 × 4–6 | Heart shaped | n/a | 4–7.5 × 3–5 | Obovate to clavate, finely striate, pale brown | n/a | n/a | + | + | + | + | − | − |
| Gamsia | |||||||||||||||
| G. aggregata | − | + | n/a | n/a | n/a | 8–10.5 × 3.5–5 | Ellipsoidal, rounded or appiculate/hyaline (2-celled) | 4–7.5 × 3.5–5 | Oval to broadly ellipsoidal, smooth, dark brown | + | + | + | + | − | − |
| G. columbina | − | + | n/a | n/a | n/a | 5–10.5 × 2.5–5.5 | Oval, smooth/hyaline (1–2-celled) | 6–13 × 3.5–6.5 | Oval to ellipsoidal, smooth, dark brown | + | + | + | + | − | − |
| Wardomyces | |||||||||||||||
| W. anomalus | − | + | n/a | n/a | n/a | n/a | n/a | 4–8 × 3.5–6 | Oval, smooth, dark brown | + | + | + | + | − | − |
| W. giganteus | + | + | 4–5.5 × 3.5–4 | Reniform (2 germ pores) | n/a | n/a | n/a | 6.5–14 × 3.5–5 | Ellipsoidal, smooth, dark brown | + | + | + | − | − | − |
| W. humicola | − | + | n/a | n/a | n/a | n/a | n/a | 9–12 × 2.5–5.5 | Navicular, smooth, dark brown | n/d | n/d | n/d | n/d | n/d | n/d |
| W. inflatus | − | + | n/a | n/a | n/a | n/a | n/a | 6–8 × 3.5–5 | Ellipsoidal to cylindrical, smooth, dark brown | + | + | + | + | − | − |
| W. ovalis | − | + | n/a | n/a | n/a | 5.5–10 × 3.5–6 | Oval, smooth/hyaline to subhyaline (1-celled) | 7–11 × 4–5 | Ellipsoidal, smooth, dark brown | + | + | + | + | − | − |
| W. pulvinatus | − | + | n/a | n/a | n/a | n/a | n/a | 5.5–10 × 3–4.5 | Navicular, smooth, dark brown | + | + | + | + | − | − |
| Wardomycopsis | n/a | n/a | n/a | n/a | n/a | n/a | n/a | ||||||||
| Ws. humicola | − | + | n/a | n/a | n/a | 4–5 × 2.5–3 | Ovate to cylindrical, smooth, smokey brown | n/a | n/a | − | + | + | + | − | − |
| Ws. inopinata | + | + | 3–3.5 × 2.5–3 | Reniform to triangular (1 germ pore) | n/a | 4–5.5 × 4–5.5 | Globose to subglobose, smooth, olive-brown | n/a | n/a | n/d | n/d | n/d | n/d | n/d | n/d |
| Ws. litoralis | − | + | n/a | n/a | n/a | 5–7 × 3–4.5 | Obovoid to broadly ellipsoidal, smooth, dark olive brown, | n/a | n/a | − | + | + | + | + | + |
n/a, not available; n/d, not determined.
Abbott (2000) considered this species a synonym of C. purpureofuscus s. lat. based on a broad range of variation in the ornamentation patterns of the conidia seen by light microscopy and SEM. Our phylogenetic results, however, showed that the two are not conspecific and can be easily differentiated by the morphology of their conidia. The conidia of C. asperulum (CBS 582.71) are apically pointed and coarsely roughened with a spiral-sculpted appearance, while those of C. purpureofuscum (UAMH 9209) are smooth to finely roughened with a slender pointed apex.
Cephalotrichum brevistipitatum Sandoval-Denis, Guarro & Gené, sp. nov. MycoBank MB814530. Fig. 8.
Fig. 8.
Cephalotrichum brevistipitatum (ex-type CBS 157.57). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–F. Synnemata. G. Conidiogenous cells. H–I. Conidia. Scale bars: D–F = 100 μm; G–I = 5 μm.
Etymology: From the Latin words brevis-small and stipes-tree trunk, “short-stiped”, referring to the short synnemata.
Colonies on OA and PCA reaching 47–50 mm diam in 14 d at 25 °C, flat, velvety with scarce aerial mycelium, front and reverse golden grey (4C2). On PDA reaching 31–33 mm diam in 14 d at 25 °C, radially folded, velvety to felty, olive-brown (4D3/4E3), with regular margin; reverse olive-brown (4D3). Hyphae septate, hyaline to pale brown, smooth- and thin-walled, 1.5–4 μm wide. Conidiophores unbranched or sparingly branched, often consisting of single annellides borne sessile on the aerial hyphae or in groups of 2–3 annellides on short basal cells, 4–5 × 3–4 μm, pale brown, smooth- and thin-walled, usually forming synnemata. Synnemata 300–500 μm high, stipes pale brown to brown, 9–14 μm wide, conidial heads brown, subglobose, ellipsoidal or short clavate; setae absent. Annellides ampulliform, 6–9 × 2.5–3.5 μm, subhyaline to pale brown, smooth- and thin-walled. Conidia ellipsoidal, 6–7 × 3.5–4 μm, with truncate base and rounded apex, pale brown, smooth- and thin-walled, arranged in long chains.
Cardinal temperatures for growth — Optimum 25–30 °C, maximum 35 °C, minimum 15 °C.
Material examined: The Netherlands, Wageningen, from Solanum tuberosum, 1957, PD A-1379 (Holotype CBS H-22332; culture ex-type CBS 157.57).
Notes: Cephalotrichum brevistipitatum is morphologically similar to C. purpureofuscum. However, the latter species has larger synnemata (800–1600 μm high) with compact black stipes and apically pointed conidia. Cephalotrichum brevistipitatum has conidia with rounded apices and small synnemata, up to 500 μm high, with brown stipes formed by somewhat loose, pale brown hyphae.
Cephalotrichum columnare (H.J. Swart) S.P. Abbott, comb. nov. MycoBank MB814969. Fig. 9.
Fig. 9.
Cephalotrichum columnare (ex-type CBS 159.66). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–E. Conidiophores. F–H. Conidiogenous cells and conidia. Scale bars: D–H = 5 μm.
Basionym: Doratomyces columnaris H.J. Swart, Acta Bot. Neerl. 15: 521. 1967.
Material examined: South Africa, Johannesburg, Melville Koppies Nature Reserve, from dung of Lepus, 1964, H.J. Swart (Holotype IMI 116691; culture ex-type CBS 159.66).
Descriptions and illustrations: Swart, 1967, Abbott, 2000.
Notes: Synnemata are more reduced than in most other species of Cephalotrichum. Abbott (2000) suggested a morphological similarity to synnemata seen in asexual morphs of Kernia species (described as Scopulariopsis morphs) and some Graphium species, but molecular data confirm a close relationship between C. columnare and other species in Cephalotrichum (Fig. 1). In the study of Abbott (2000), several isolates of C. columnare did not produce synnemata in culture and recent isolations of this species from indoor environments show a propensity of synnema production to be reduced or disappear after primary isolation and overall sporulation to be sparse. We were also unable to obtain synnemata from the ex-type culture (CBS 159.66) in this study; however, the isolate produced dry conidia in chains characteristic of Cephalotrichum instead to conidia in slimy heads typical of Graphium and Kernia asexual morphs (Lackner et al. 2014).
Cephalotrichum columnare morphologically resembles C. brevistipitatum and C. microsporum. However, the conidia of C. brevistipitatum are pale brown and smooth to finely roughened (6–7 × 3.5–4 μm), while those of C. microsporum are brown and smaller (3.5–5 × 2–3 μm). In addition, these two species have colonies with a faster growth rate (47–50 mm and 26–37 mm diam, respectively, in 14 d at 25 °C). By contrast, C. columnare produces asymmetrical, dark brown, smooth-walled conidia (5.5–7.5 × 2.5–4 μm) and slow-growing colonies (18–19 mm diam in 14 d at 25 °C) with poorly developed synnemata.
Cephalotrichum cylindricum (Clem. & Shear) S. P. Abbott, comb. nov. MycoBank MB814970, Fig. 10.
Fig. 10.
Cephalotrichum cylindricum (ex-epitype UAMH 1348). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–E. Synnemata. F. Detail of the apical part of a synnema. G. Detail of a synnemal seta. H–I. Conidiogenous cells. J–K. Conidia. Scale bars: D–E = 200 μm; F = 100 μm; G = 20 μm; H–K = 5 μm.
Basionym: Trichurus cylindricus Clem. & Shear, in Pound & Clements, Bot. Surv. Nebr. 4: 7. 1896.
Synonym: Trichurus terrophilus Swift & Povah, Mycologia 21: 214. 1929, non Cephalotrichum terricola Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 221. 2011.
Material examined: USA, Lincoln, Nebraska, on decaying seeds of Cucurbita maxima, 1895, collector unknown (Holotype NEB0041953). Epitype designated here: USA, Kansas, seed of Sorghum, 1955, C.T. Rogerson, MBT-203075 (culture ex-epitype UAMH 1348). South Africa, Bekker, timber of Eucalyptus saligna, 1951, TRL8-FPRL (CBS 448.51).
Description and illustrations: Swift, 1929, Abbott, 2000.
Notes: Our phylogenetic and morphological results support the designation of the epitype culture selected by Abbott (2000), which is formally proposed here. Only three species of Cephalotrichum produce setae in the upper part of the synnemata, i.e., C. cylindricum, C. dendrocephalum, and C. gorgonifer. Cephalotrichum cylindricum can be differentiated by the production of straight, unbranched or branched setae on synnemata 450–700 μm tall with brown stipes. By contrast, C. dendrocephalum and C. gorgonifer, produce undulating and spirally twisted setae, respectively, and synnemata >1 000 μm tall with dark brown to black stipes.
Cephalotrichum dendrocephalum (Udagawa, Y. Horie & Abdullah) S.P. Abbott, comb. nov. MycoBank MB814971. Fig. 11
Fig. 11.
Cephalotrichum dendrocephalum (ex-isotype CBS 528.85). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–E. Synnemata. F. Detail of synnemal setae. G. Conidiogenous cells. H. Conidia. Scale bars: D–E = 200 μm; F–H = 5 μm.
Basionym: Trichurus dendrocephalus Udagawa, Y. Horie & Abdullah, Mycotaxon 23: 253. 1985.
Material examined: Iraq, near Basrah, cultivated soil from date palm plantation, 1983, S.K. Abdullah (Holotype NHL 2927; culture ex-isotype CBS 528.85).
Description and illustrations: Udagawa et al., 1985, Abbott, 2000.
Notes: The presence of characteristic undulating branched setae on large synnemata is a distinctive morphological characteristic of this species (see notes on C. cylindricum). In the absence of setae, C. dendrocephalum can be confused with C. purpureofuscum. However, C. dendrocephalum exhibits brown to grey conidia, measuring 5–7 × 2.5–3.5 μm, with rounded or pointed apex, and grey colonies with a growth rate 18–39 mm diam in 14 d at 25 °C; while C. purpureofuscum produces somewhat larger (5–8 × 3–4.5 μm) green-brown pointed conidia, and dark grey to black colonies with a faster growth rate (44–56 mm diam in 14 d at 25 °C).
Cephalotrichum gorgonifer (Bainier) Sandoval-Denis, Gené & Guarro, comb. nov. MycoBank MB817599. Fig. 12.
Fig. 12.
Cephalotrichum gorgonifer (ex-epitype CBS 635.78). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–E. Synnemata. F. Detail of a synnemal seta. G. Conidiogenous cells. H–I. Conidia. Scale bars: D–E = 200 μm; F–I = 10 μm.
Basionym: Trichurus gorgonifer Bainier, Bull. Soc. Mycol. France. 23: 230. 1907.
Synonyms: Trichurus spiralis Hasselbr., Bot. Gaz. 29: 321. 1900.
Cephalotrichum heliciforme T.Y. Zhang, Mycosystema 33: 948. 2014, non Cephalotrichum spirale H.M. Liu, H.Q. Pan & T.Y Zhang, Mycotaxon 117: 220. 2011.
Material examined: Lectotype designated here: T. XXXIII, plate XXV in Bainier G. Mycothèque de l'École de Pharmacie, XXI-XXIII. Bulletin de la Société Mycologique de France, 1907, 23: 218–241, MBT-372236. Canada, Alberta, Spruce Grove, steamed decomposing mushroom compost, unknown date, L. Sigler (UAMH 3585). South Africa, from unknown origin, 1953, unknown collector (CBS 368.53). Epitype designated here: The Netherlands, from human hair, 1978, S.S.D.Z Delft, MBT-203078 (CBS H-22697, culture ex-epitype CBS 635.78). USA, from unknown origin, 1908, A.F. Blakeslee (CBS 131.08); from bronchoalveolar lavage fluid, unknown date, D.A. Sutton (UTHSCSA DI14-63 = FMR 13444); from bronchoalveolar lavage fluid, unknown date, D.A. Sutton (UTHSCSA DI14-64 = FMR 13445); from bronchoalveolar lavage fluid, unknown date, D.A. Sutton (UTHSCSA DI14-69 = FMR 13450); from maxillary sinus fluid, unknown date, D.A. Sutton (UTHSCSA DI14-71 = FMR 13452); from bronchoalveolar lavage fluid, unknown date, D.A. Sutton (UTHSCSA DI14-75 = FMR 13456).
Description and illustrations: Ellis, 1971, Domsch et al., 2007.
Notes: Zhang et al. (2014) proposed Cephalotrichum heliciforme as nomen novum for Trichurus spiralis Hasselbr. to avoid nomenclatural conflict with the recently described species Cephalotrichum spirale by Jiang et al. (2011), which was characterised by the spiral pattern of roughness on the conidial surface. However, according to the International Code of Nomenclature (ICN) for algae, fungi and plants, a new combination is required for C. heliciforme as there is an older epithet available for this species (Trichurus gorgonifer). Therefore, the new combination C. gorgonifer is proposed and C. heliciforme is reduced to a synonym. Because type material for T. gorgonifer is unexistent, an illustration included in the protologue reproduced here (Fig. 13) serves as lectotype of C. gorgonifer. In addition, to asure the availability of information for modern identification, an epitype culture is designated.
Fig. 13.
Reproduction of the original drawings by Bainier (1907) illustrating Trichurus gorgonifer (original numbers are maintained to indicate the different structures). 1–2. Hyphae and penicillate conidiophores. 3. Young synnema showing setae. 4. Mature synnemata. 5. Abnormal synnema. 6. Conidia.
The widespread species C. gorgonifer has been commonly known as T. spiralis, and it is a common inhabitant of soil and decaying vegetable material. However, the majority of isolates included in this study were from human clinical samples, mainly hair and respiratory specimens. Although C. gorgonifer is able to grow at human physiological temperature, the potential pathogenic role of this species is uncertain since no clinical data are available.
Cephalotrichum gorgonifer is morphologically similar to C. cylindricus and C. dendrocephalus, but it is easily recognisable by its spirally coiled setae. Strains with poorly developed or lacking synnematal setae could be confused with C. purpureofuscum, however the conidia of the latter species are brown with slightly pointed apices, while those of C. gorgonifer are grey-brown with rounded apices.
Cephalotrichum hinnuleum Sandoval-Denis, Guarro & Gené, sp. nov. MycoBank MB814531. Fig. 14.
Fig. 14.
Cephalotrichum hinnuleum (ex-type CBS 289.66). A–D. Colonies on PCA, OA and PDA, respectively, and pigment production on PDA after 14 d at 25 °C. E–G. Synnemata. H. Detail of the apical portion of a synnema. I. Annellidic conidiogenous cells and conidia. J–K. Polyblastic conidiogenous cells and conidia. Scale bars: E–G = 200 μm; H–K = 5 μm.
Etymology: From the Latin hinnuleus-fawn, referring to the brown “fawn” colour of the colony reverse.
Colonies on OA and PCA reaching 32–38 mm diam in 14 d at 25 °C, flat, velvety to floccose with a regular margin, obverse and reverse brown-grey to olivebrown (4F2/4F3). On PDA reaching 29–30 mm diam in 14 d at 25 °C, velvety to felty, golden grey to brown-grey (4C2/D2) with regular margin; reverse at first golden grey to brown-grey (4C2/D2), turning pale brown to brown (6D7/6E7) with age by the production of a non-diffusible pigment. Hyphae septate, subhyaline to pale brown, smooth- and thin-walled, 2–4 μm wide. Conidiophores branched, septate, 12–19 × 2–3 μm, pale brown, smooth- and thin-walled, commonly aggregated in dense synnemata. Synnemata 800–1 600 μm high, stipes compact, dark brown to black, 10–30 μm wide, conidial heads grey, clavate to ellipsoidal; setae absent. Annellides ampulliform to cylindrical, 5.5–9 × 2–3.5 μm, subhyaline to pale brown, smooth- and thin-walled. Conidia subglobose to ellipsoidal, 6–7.5 × 2.5–4 μm with truncate base and pointed apex, pale brown, smooth- and thin-walled, arranged in long chains. An echinobotryum-like synasexual morph can be present, producing conidia from short penicillate conidiophores, 10–15 × 2.5–3 μm, on the top of synnemata or on the hyphae; conidia oval to navicular, 8.5–10 × 5.5–7 μm, with truncate base and pointed apex, dark brown, coarsely verrucose, thick-walled.
Cardinal temperatures for growth — Optimum 15–25 °C, maximum 30 °C, minimum 5 °C.
Material examined: Australia, Tasmania, from dung of deer, 1963, K. Tubaki (Holotype CBS H-22333; culture ex-type CBS 289.66).
Notes: Cephalotrichum hinnuleum and C. stemonitis are the only species of the genus producing an echinobotryum-like synasexual morph. The former species is easily distinguished by its smaller (8–10 × 5.5–7 μm versus 8–19 × 6–7.5 μm in C. stemonitis) and unbeaked, echinobotryum-like conidia. In addition, the most striking feature of this new species is the presence of a non-diffusible brown pigment in the colony reverse on PDA.
Cephalotrichum microsporum (Sacc.) P.M. Kirk, Kew Bull. 38: 578. 1984. Fig. 15.
Fig. 15.
Cephalotrichum microsporum (ex-epitype CBS 523.63). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–F. Synnemata. G. Detail of the apical portion of synnema. H–I. Conidia. Scale bars: D–F = 200 μm; G–I = 5 μm.
Basionym: Stysanus microsporus Sacc., Michelia. 1: 274. 1878.
Synonyms: Doratomyces microsporus (Sacc.) F.J. Morton & G. Sm., Mycol. Pap. 86: 77. 1963.
Graphium graminum Cooke & Massee, Grevillea. 16: 11. 1887.
Graphium pistillarioides Speg., Revista Fac. Agron. Univ. Nac. La Plata 2: 254. 1896.
? Cephalotrichum inflatum Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 213. 2011.
? Cephalotrichum ovoideum Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 217. 2011.
? Cephalotrichum robustum Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 218. 2011.
Material examined: Canada, Alberta, near Peace River, indoor air of home, 1998, S.P. Abbott (UAMH 9365). Epitype designated here: Germany, Schleswig-Holstein, Kiel-Kitzeberg, wheat-field soil, 1963, W. Gams, MBT-203079 (CBS H-12123, culture ex-epitype CBS 523.63). Italy, Selva, in rotting trunk of Robinia pseudacacia, Aug. 1875, P.A. Saccardo (Holotype PAD 663).
Descriptions and illustrations: Morton and Smith, 1963, Ellis, 1971, Domsch et al., 2007.
Notes: This is one of the most commonly isolated species of Cephalotrichum and has been studied as a potential source of keratinases for industrial applications (Gradisar et al., 2000, Hublin et al., 2002). Abbott (2000) examined numerous isolates of this species from diverse geographical origins and its morphological observations agree with ours. In order to fix the name of this taxon, we have selected the strain CBS 523.63 as epitype. Cephalotrichum microsporum is morphologically similar to C. purpureofuscum. However, C. microsporum produces synnemata 500–1 000 μm long, smooth conidia measuring 3.5–5 × 2–3 μm, and grey colonies, while C. purpureofuscum has larger synnemata (up to 1 600 μm long), smooth to finely roughened, larger conidia (5–8 × 3–4.5 μm) and has dark grey to black colonies.
Cephalotrichum nanum (Ehrenb.) S. Hughes, Canad. J. Bot. 36: 744. 1958. Fig. 16.
Fig. 16.
Cephalotrichum nanum (ex-epitype CBS 191.61). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–F. Synnemata. G. Detail of the apical portion of synnema. H–I. Conidia. Scale bars: D–F = 200 μm; G–I = 10 μm.
Basionym: Periconia nana Ehrenb., Sylv. mycol. berol. (Berlin) 13: 24. 1818.
Synonyms: Stilbum nanum (Ehrenb.) Spreng., Syst. veg., Edn 16. 4: 547. 1827.
Graphium nanum (Ehrenb.) Sacc., Syll. Fung. 4: 616. 1886.
Doratomyces nanus (Ehrenb.) F.J. Morton & G. Sm., Mycol. Pap. 86: 80. 1963.
Stysanus stemonitis (Pers.: Fr.) var. fimetarius P. Karst. [as ‘stemonites’], Meddel. Soc. Fauna Fl. Fenn. 14: 93. 1887.
Stysanus fimetarius (P. Karst.) Massee & E.S. Salmon, Ann. Bot. 16: 86. 1902.
Stysanus verrucosus Oudem., Ned. Kruidk. Arch. 2: 923. 1903.
Material examined: Canada, Alberta, Elk Island National Park, dung of bison, 1997, S.P. Abbott (UAMH 9126). Lectotype designated here: Unknown country, on leafs of Pinus strobus, unknown date, C.G. Ehrenberg, MBT-372237 (L0111516). Epitype designated here: England, Surrey, Richmond Park, dung of deer, 1956, J. Hawkins, MBT-203082 (CBS H-22698, culture ex-epitype CBS 191.61).
Descriptions and illustrations: Morton & Smith (1963), Ellis (1971), Domsch et al. (2007).
Notes: Cephalotrichum nanum is a common species on dung. This species is distinguished by its large, globose to subglobose and coarsely warted conidia, 6–8.5 × 4.5–7.5 μm, which resemble those of Scopulariopsis brevicaulis, from which it clearly differs in the colony colour (dark grey-brown, turning black-grey in C. nanum, tan in S. brevicaulis) and in the production of well-developed, black synnemata. Cephalotrichum asperulum is a further similar species, but its conidia are narrower (5–8.5 × 3–4 μm), oval to ellipsoidal and finer roughening.
According to Hughes (1958) and Seifert (1985), Ehrenberg's herbarium material of Periconia nana was deposited in B, DAOM and L. However, material in B does not exist anymore as is presumed to be lost during the Second World War (Dr. Robert Lücking, pers. comm.). Original material was located in L and, in order to stabilise the use of the name, it is designated here to serve as lectotype. In addition, the species is epitypified with the strain CBS 191.61, which matches with the species concept by Hughes (1958).
Cephalotrichum purpureofuscum (Schwein.: Fr.) S. Hughes, Canad. J. Bot. 36: 744. 1958. Fig. 17.
Fig. 17.
Cephalotrichum purpureofuscum (UAMH 9209). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–E. Synnemata. F. Detail of the apical portion of synnema. G. Conidiophores and conidiogenous cells. H–I. Conidia. Scale bars: D–E = 200 μm; F–I = 10 μm.
Basionym: Aspergillus purpureofuscus Schwein., Trans. Amer. Philos. Soc. 4: 282. 1832: Fr., Syst. mycol. (Lundae) 3: 388, Index: 53. 1832.
Synonyms: Stysanus purpureofuscus (Schwein.) S. Hughes, Canad. J. Bot. 31: 615. 1953.
Doratomyces purpureofuscus (Schwein.) F.J. Morton & G. Sm., Mycol. Pap. 86: 74. 1963.
Stilbum pusillum Wallr., Fl. crypt. Germ. (Norimbergae) 2: 326. 1833.
Graphium pusillum (Wallr.) Sacc., Syll. Fung. 4: 614. 1886.
Ceratopodium pusillum (Wallr.) Kuntze, Revis. gen. pl. (Leipzig) 2: 847. 1891.
Stilbum brevipes Wallr., Fl. crypt. Germ. (Norimbergae) 2: 326. 1833.
Sporocybe brevipes (Wallr.) Sacc., Syll. Fung. 4: 607. 1886.
Cephalotrichum brevipes (Wallr.) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Cephalotrichum leucocephalum Wallr., Fl. crypt. Germ. (Norimbergae) 2: 330. 1833.
Graphium leucocephalum (Wallr.) Sacc., Syll. Fung. 4: 615. 1886.
Pachnocybe grisea Berk., in Smith, Engl. Fl., Fungi (Edn 2) (London) 5: 334. 1836.
Graphium griseum (Berk.) Sacc., Syll. Fung. 4: 616. 1886.
Periconia fusca Corda, Icon. fung. (Prague) 1: 19. 1837.
Stysanus fuscus (Corda) E.W. Mason & M.B. Ellis, Mycol. Pap. 56: 31. 1953.
Stysanus mandlii Mont., Ann. Sci. Nat., Bot. 4: 365. 1845.
Stysanus stemonitis (Pers.) Corda formae mandlii (Mont.) Guég., Bull. Soc. Mycol. France. 19: 219. 1903.
Periconia discolor Corda, Icon. fung. (Prague) 3: 13. 1839.
Periconia brassicicola Berk. & Broome [as ‘brassicaecola’], Ann. Mag. Nat. Hist. 15: 33. 1875.
Sporocybe brassicicola (Berk. & Broome) Sacc. [as ‘brassicaecola’], Syll. Fung. 4: 606. 1886.
Cephalotrichum brassicicola (Berk. & Broome) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Stysanus medius Sacc., Michelia 2: 300. 1881.
Stysanopsis media (Sacc.) Ferraris, Ann. Mycol. 7: 281. 1909.
Cephalotrichum medium (Sacc.) S. Hughes, Canad. J. Bot. 36: 744. 1958.
Pycnostysanus medius (Sacc.) Bat. & Peres, Nova Hedwigia 2: 469. 1960.
Doratomyces medius (Sacc.) Matsush., Matsush. Mycol. Mem. 1: 33. 1980.
Sporocybe byssoides (Pers.) Bon.: Sacc., Syll. Fung. 4: 606. 1886.
Sporocybe sacchari Speg., Revista Fac. Agron. Univ. Nac. La Plata 2: 253. 1896.
? Cephalotrichum longicollum Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 213. 2011.
? Cephalotrichum macrosporum Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 214. 2011.
? Cephalotrichum oblongum J.J. Xu & T.Y. Zhang, Mycotaxon 117: 216. 2011.
? Cephalotrichum terricola Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 221. 2011.
Material examined: Canada, British Columbia, Pemberton, Indoor air of school library, 1998, S.P. Abbott (UAMH 9209).
Descriptions and illustrations: Morton & Smith (1963), Ellis (1971), Domsch et al. (2007).
Notes: No single morphological feature distinguishes this commonly reported species, and it can more easily be described as lacking distinctive characters than defined by recognisable features such as setae, echinobotryum-like synasexual morph roughened conidia, or spores of particularly large or small dimensions. Not surprisingly, it has been described in the literature on a number of occasions. Here, of the four isolates originally received as C. purpureofuscum (Table 1), only that studied by Abbott (2000) could correspond to such species (UAMH 9209); the other three have been reidentified as C. brevistipitatum, C. gorgonifer and C. microsporum. Cephalotrichum purpureofuscum is morphologically similar to C. cylindricum and C. gorgonifer, all having similar oval to ellipsoidal, brown conidia, and synnemata of similar size. However, the absence of setae is the most relevant distinctive feature of C. purpureofuscum. Also, its conidia are slightly larger (5–8 × 3–4.5 μm) and smooth to finely roughened, while those of C. cylindricum and C. gorgonifer are always smooth and measure 4.5–6 × 2.5–3.5 μm and 4–8 × 2.5–4 μm, respectively. The absence of an echinobotryum-like state easily separates this species from C. stemonitis.
Several recently described species (i.e., C. longicollum, C. macrosporum, C. oblongum and C. terricola) are here considered probable synonyms of C. purpureofuscum based on their morphological similarity and molecular comparisons of ITS sequences available in GenBank (see notes on doubtful species).
Abbott (2000) studied a large set of isolates of C. purpureofuscum from different substrates and geographic origins, and selected a putative ex-epitype culture, however, it was not formally proposed. The species concept presented and illustrated here centres on UAMH 9209, which was also characterised based on DNA sequence data. However, considering that we have not had access to the type material (BPI) and that the species seems not to be properly characterised, no epitype is designated at the moment until additional isolates can be morphologically and molecularly analysed for a correct circumscription of the species.
Cephalotrichum stemonitis (Pers.: Fr.) Nees, Mag. Ges. Naturf. Freunde Berlin 3: 20. 1809. Fig. 18.
Fig. 18.
Cephalotrichum stemonitis (ex-neotype CBS 103.19). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–E. Synnemata. F. Tip of synnema with annellidic conidiogenous cells and conidia. G–H. Polyblastic conidiogenous cells and conidia. I. Conidia of both kinds. Scale bars: D–E = 500 μm; F–I = 10 μm.
Basionym: Isaria stemonitis Pers., Comm. fung. clav. (Lipsiae): 234. 1797.
Synonyms: Periconia stemonitis (Pers.) Pers., Syn. meth. fung. (Göttingen) 2: 687. 1801.
Cephalotrichum stemonitis (Pers.: Fr.) Link, Mag. Ges. Naturf. Freunde Berlin 3: 20. 1809.
Stysanus stemonitis (Pers.: Fr.) Corda, [as ‘stemonites’], Icon. fung. (Prague) 1: 22. 1837.
Doratomyces stemonitis (Pers.) F.J. Morton & G. Sm., Mycol. Pap. 86: 70. 1963.
Periconia stemonitis (Pers.) Pers. var. communis Alb. & Schwein., Consp. fung. (Leipzig): 358. 1805.
Periconia stemonitis (Pers.) Pers. var. pusilla Alb. & Schwein., Consp. fung. (Leipzig): 358. 1805.
Periconia subulata Nees, Nova Acta Acad. Leop. Carol. Ac. Naturf. Fo. 9: tab. 5, fig. 8. 1818.
Stilbum subulatum (Nees) Spreng., Syst. veg., 16th ed. 4: 547. 1827.
Pachnocybe subulata (Nees) Berk., In Smith, Engl. Fl., Fungi, 2nd ed., 5: 333. 1836.
Graphium subulatum (Nees) Sacc., Syll. Fung. 4: 612. 1886. [nom. illegit., Art. 53.1, non Pass. & Beltrani 1882]
Ceratopodium subulatum (Nees) Kuntze, Rev. Gen. Pl. 2: 847. 1891.
Doratomyces neesii Corda, Sturm, Deutschl. Fl., Abt. 3 (Pilze Deutschl.) 2: 65. 1829.
Echinobotryum atrum Corda, Sturm, Deutschl. Fl., 3 Abt. (Pilze Deutschl.) 3: 51. 1831.
Stilbum setosum Wallr., Fl. crypt. Germ. (Norimbergae) 2: 329. 1833.
Periconia setosa (Wallr.) Rabenh., Deutschl. Krypt.-Fl. (Leipzig) 1: 118. 1884.
Sporocybe setosa (Wallr.) Sacc., Syll. Fung. 4: 607. 1886.
Cephalotrichum setosum (Wallr.) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Stilbum typhinum Wallr., Fl. crypt. Germ. (Norimbergae) 2: 330. 1833.
Graphium typhinum (Wallr.) Sacc., Syll. Fung. 4: 617. 1886.
Ceratopodium typhinum (Wallr.) Kuntze, Revis. gen. pl. (Leipzig) 2: 847. 1891.
Echinobotryum parasitans Corda, Pracht-Flora. 17: 1839.
Stysanus capitatus Reinke & Berthold, Die Zersetzung der Kartoffel durch Pilze. 37. 1879.
Stysanus ramifer Rolland, Bull. Soc. Mycol. France. 6. 106: 1890.
Stysanus tubericola Ellis & Dearn., Proc. Canad. Inst. 1. 90: 1897.
Stysanus stemonitis (Pers.) Corda var. ramosa Pim., Trans. Brit. mycol. Soc. 1. 65: 1899.
Doratomyces stemonitis (Pers.) F.J. Morton & G. Sm var. keratinolyticus Dominik & Majchr. [as ‘keratinolytica’], Ekol. Pol. 13: 434. 1965.
Material examined: Canada, Ontario, near Guelph, soil, 1961, G.L. Barron, (UAMH 1532). Unknown country, unknown substratum, 1935, N.F. Conant (CBS 180.35). Neotype designated here: The Netherlands, Wageningen, from seed, 1919, C.M. Doyer, MBT-203081 (CBS H-12129, culture ex-neotype CBS 103.19).
Descriptions and illustrations: Morton and Smith, 1963, Domsch et al., 2007.
Notes: The main distinguishing morphological characteristic of this species is the presence of an echinobotryum-like synasexual morph with fusiform, coarsely warted and apically beaked conidia, 8–19 × 6–7.5 μm (Abbott 2000). The other species exhibiting an echinobotryum-like morph is C. hinnuleum, but C. stemonitis is different by robust synnemata of 2 000–3 000 μm tall, smooth conidia measuring 6–9 × 4–5 μm, and the shape and size of the echinobotryum-like conidia. Cephalotrichum hinnuleum has shorter synnemata 800–1 600 μm tall, narrower (6–7.5 × 2.5–4 μm), smooth to finely verruculose conidia, and the echinobotrym-like morph exhibits smaller (8.5–10 × 5.5–7 μm), oval to navicular verrucose and slightly pointed conidia. In addition, the latter species produces a non-diffusible, pale brown to brown (6D7/E7) pigment on PDA.
Only four specimens were located in the herbarium Persoon in L. However, all four are labelled as P. stemonitis, and none of them is regarded as type. Since the holotype of Isaria stemonitis seems to be lost, a neotype specimen and an ex-neotype culture are designated here to fix the use of the name.
Cephalotrichum verrucisporum (Y.L. Jiang & T.Y. Zhang) Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 224. 2011. Fig. 19
Fig. 19.
Cephalotrichum verrucisporum (CBS 187.78). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–F. Synnemata. G. Detail of the apical part of synnema. H–I. Conidiogenous cells and conidia. J–K. Conidia. Scale bars: D–E = 500 μm; F = 100 μm; G–K = 5 μm.
Basionym: Doratomyces verrucisporus Y.L. Jiang & T.Y. Zhang, Mycotaxon 104: 133. 2008.
Material examined: The Netherlands, Katwijk, from sand dune soil, 1978, W. Gams (CBS 187.78).
Description and illustration: Jiang & Zhang (2008).
Notes: Although the holotype of C. verrucisporum (HSAUP051029, preserved at the Herbarium of the Shandong Agricultural University: Plant Pathology, China) was not available for morphological comparison, in GenBank there was an ITS sequence from the ex-type strain (accession number JX537968), which had 100 % similarity with the strain CBS 187.78 examined here. The molecular and morphological data confirm this taxon as a distinct species of Cephalotrichum.
Cephalotrichum verrucisporum is morphologically similar to C. asperulum. Both species produce rough-walled conidia with a spiral-sculpted ornamentation. However, synnemata are up to 3 000 μm tall and the conidia are ovoid and darker in C. verrucisporum, whereas synnemata are up to 1 000 μm tall and conidia are oval to ellipsoidal and pale brown in C. asperulum. The latter species is also able to grow at 35 °C, whereas, according to our data, the maximum temperature for growth in C. verrucisporum is 30 °C.
Fairmania Sacc., Ann. Mycol. 4: 276. 1906.
Colonies restricted, velvety to felty with granular centre, flat, white, becoming grey-white with dark centre. Hyphae hyaline, thin- and smooth-walled. Conidiophores undifferentiated, usually unbranched and borne laterally on the hyphae, hyaline. Conidiogenous cells annellidic, short-cylindrical, subhyaline to pale brown, smooth-walled. Conidia obovoid to cylindrical, dark brown, smooth- and thick-walled with one to several longitudinal striations. Ascomata superficial or immerse, perithecial, black, hairy, often with a well-developed neck. Asci irregularly oval, evanescent, 8-spored. Ascospores 1-celled, broadly lunate, golden yellow, pale brown in mass, smooth, with a single germpore.
Type species: Fairmania singularis Sacc.
Notes: This monotypic genus differs from the other members of Microascaceae by its conidia with several longitudinal striations. Whether these striations participate in conidial germination has been controversial (Barron 1966). However, our observations showed that germination actually occurs laterally from the striations, confirming the observations by Barron (1966), that they function as germ slits.
Fairmania singularis Sacc., Ann. Mycol. 4: 276. 1906. Fig. 20.
Fig. 20.
Fairmania singularis (ex-epitype CBS 505.66). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D. Ascomata. E. Ascomatal peridium. F. Asci. G–H. Ascospores. I. Conidiogenous cells. J. Conidia. Scale bars: D = 20 μm; E–J = 5 μm.
Synonyms: Microascus singularis (Sacc.) Malloch & Cain, Canad. J. Bot. 49: 859. 1971.
Microascus doguetii Moreau, Rev. Mycol. (Paris). 18: 174. 1953.
Material examined: USA, Lyndonville, New York, on rotten wood of Fagi americanae, C.E. Fairman (Holotype PAD1239). Epitype designated here: USA, Maine, Kittery Point, from barrel bottom, 1966, R. Thaxter, MBT-202772 (culture ex-epitype CBS 505.66). Canada, Toronto, from unknown substrate, 1964, M. Corlett (CBS 249.64). Japan, Tokyo, laboratory contaminant, 1962, S. Udagawa (CBS 414.64).
Descriptions and illustrations: Barron et al., 1961, Udagawa, 1963, Arx et al., 1988.
Notes: Malloch & Cain (1971) studied Saccardo's original material of F. singularis and concluded that it is morphologically identical to M. doguetii. Both species were then synonymised and placed in Microascus, M. singularis having priority. This synonymy was also accepted by von Arx et al. (1988).
However, the longitudinal striations in the conidial wall and presence of erect and thick-walled annellides are singular features of this species, which are absent in all genera of Microascaceae, and considered here of taxonomic value for the reintroduction of this obscure genus. Previous phylogenetic analyses supported such morphological differences (Issakainen et al., 2003, Sandoval-Denis et al., 2016).
Gamsia M. Morelet, Ann. Soc. Sci. Nat. Archéol. Toulon Var 21: 105. 1969.
Colonies grey to black, compact and slow growing. Hyphae mostly superficial, hyaline, thin- and smooth-walled. Conidiophores mostly undifferentiated, unbranched or sometimes once or twice branched, borne laterally on the hyphae, septate, hyaline, smooth-walled. Conidiogenous cells and conidia of two types: i) conidiogenous cells polyblastic, cylindrical with a swollen apical part, hyaline, smooth-walled; conidia borne solitary in lateral succession and forming large apical clusters, 1-celled, ovoid to broadly ellipsoidal, flat at the base, brown to black, smooth- and thick-walled, often with a longitudinal germ slit; ii) conidiogenous cells annellidic, sometimes grouped in sporodochia, subulate to cylindrical, hyaline, smooth-walled; conidia catenate, 1–2-celled, oval to ellipsoidal, truncate at the base, hyaline, smooth- and thin-walled.
Type species: Gamsia columbina (Demelius) Sandoval-Denis, Guarro & Gené.
Notes: The genera Gamsia and Hennebertia were simultaneously erected to accommodate those Wardomyces species that have 1-septate annelloconidia (Morelet 1969), being competing synonyms. The selection of Gamsia was posteriorly settled when Ellis (1976) took up only this name. However, our morphological study demonstrated that the conidial septation is not a constant character in this genus. In contrast, the lack of well-differentiated conidiophores, and the conidial arrangement with large apical clusters which resemble the echinobotryum-like synasexual morphs of Cephalotrichum more than those of Wardomyces, justifies the separation of the two genera, which is also supported by phylogenetic results.
Gamsia aggregata (Malloch) Kiffer & M. Morelet, Ann. Soc. Sci. Nat. Archéol. Toulon Var 47: 93. 1995. Fig. 21.
Fig. 21.
Gamsia aggregata (ex-isotype CBS 251.69). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–F. Conidiophores, polyblastic conidiogenous cells and conidia. G–K. Conidiophores, annellidic conidiogenous cells and conidia. Scale bars: D–K = 5 μm.
Basionym: Wardomyces aggregatus Malloch, Can. J. Bot. 48: 883. 1970.
Synonym: ? Gymnodochium fimicola Massee & Salmon, Ann. Bot. 16: 89. 1902.
Material examined: USA, Michigan, Emmet Co., Wycamp Lake, from dung of carnivore, 1967, D.W. Malloch (Holotype TRTC 45325; culture ex-isotype CBS 251.69).
Description and illustrations: Malloch (1970b).
Notes: This species can be easily recognised by its broadly ellipsoidal to ovoid solitary conidia, measuring 4–7.5 × 3.5–5 μm, and having a rounded apex. It also produces abundant sporodochia composed of annellides bearing 2-celled, hyaline, ellipsoidal conidia of 8–10.5 × 3.5–5 μm. The other species of the genus, G. columbina, has larger (6–13 × 3.5–6.5 μm), oval to ellipsoidal, solitary conidia with slightly pointed apices, its annellides are solitary or grouped in sporodochia, and form 1–2-celled, oval annelloconidia of 5–10.5 × 2.5–5.5 μm.
Gamsia columbina (Demelius) Sandoval-Denis, Guarro & Gené, comb. nov. MycoBank MB814578, Fig. 22.
Fig. 22.
Gamsia columbina (ex-epitype CBS 233.66). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D. Sporodochium and annellidic conidiogenous cells. E. Aseptate annelloconidia. F. Septate annelloconidia from CBS 546.69. G–H. Polyblastic conidiogenous cells and conidia. Scale bars: D–H = 10 μm.
Basionym: Trichosporum columbinum Demelius, Verh. Zool.-Bot. Ges. Wien 72: 105. 1923.
Synonyms: Wardomyces columbinus (Demelius) Hennebert, Trans. Brit. mycol. Soc. 51: 753. 1968.
Hennebertia columbina (Demelius) M. Morelet, Ann. Soc. Sci. Nat. Archéol. Toulon Var 21: 104. 1969.
Wardomyces simplex Sugiy., Y. Kawas. & H. Kurata, Bot. Mag. Tokyo 81: 244. 1968.
Gamsia simplex (Sugiy., Y. Kawas. & H. Kurata) Arx, Gen. Fungi Sporul. Cult., Edn 3: 340. 1981.
Wardomyces dimerus W. Gams, Trans. Brit. mycol. Soc. 51: 800. 1968.
Gamsia dimera (W. Gams) M. Morelet, Ann. Soc. Sci. Nat. Archéol. Toulon Var 21: 105. 1969.
Material examined: Austria, Vienna, in plum gelatin, Mar. 1917, P. Demelius (Holotype W1134). Epitype designated here: Germany, Giessen, from sandy soil, 1964, A. von. Klopotek, MBT-202773 (culture ex-epitype CBS 233.66); Schleswig-Holstein, Kiel-Kitzeberg, from wheat-field soil, 1966, W. Gams (ex-type culture of Wardomyces dimerus CBS 235.66). Japan, Osaka Region, from milled Oryza sativa, 1968, J. Sugiyama (ex-isotype culture of Wardomyces simplex CBS 546.69). The Netherlands, Wageningen, from sandy soil, 1982, J.A. van Veen (CBS 230.82).
Descriptions and illustrations: Gams, 1968, Hennebert, 1968 and Sugiyama et al., 1968, Sugiyama et al., 1969.
Notes: This species has been isolated from air, soil and decaying wood (Gams, 1968, Ellis, 1976, Domsch et al., 2007), and was associated with loss of weight and tensile strength in maple-wood (Domsch et al. 2007).
Several authors have discussed the relationship between the species originally included in Wardomyces, i.e. W. columbinus, W. dimerus, W. ovalis and W. simplex (Hennebert, 1968, Gams, 1968, Morelet, 1969, Sugiyama et al., 1969), all forming two types of conidia in culture: one dark, thick-walled and with a longitudinal germ slit, arising singly on the conidiogenous cells, and the other with hyaline, thin-walled scopulariopsis-like annelloconidia. Morelet (1969) proposed the genera Gamsia and Hennebertia to include species with septate and aseptate annelloconidia, respectively. Nevertheless, neither Gamsia nor Hennebertia have been widely accepted (Ellis, 1971, Ellis, 1976, Domsch et al., 2007, Whitton et al., 2012). Of these two competing simultaneously published synonyms only Gamsia was taken up, e.g. by Ellis (1971). Our molecular phylogeny shows that W. columbinus, a species described with only aseptate annelloconidia, belongs to the same clade as W. dimerus and W. simplex. Moreover, Gams (1968) and Sugiyama et al. (1969) reported both aseptate and septate annelloconidia in cultures of W. dimerus and W. simplex. Conidial septation thus is not a reliable criterion for generic delimitation of Gamsia. The species name W. columbinus has priority over the latter W. dimerus and W. simplex, and since only dried type material is available for this species we have selected an ex-epitype culture from the authentic material of W. columbinus studied by Hennebert (1968).
Gamsia columbina is morphologically very similar to G. aggregata, but can be distinguished by having larger and pointed solitary conidia (see notes on G. aggregata).
Wardomyces F.T. Brooks & Hansf., Trans. Brit. mycol. Soc. 8: 137. 1923.
Type species: Wardomyces anomalus F.T. Brooks & Hansf.
Descriptions and illustrations: Brooks and Hansford, 1923, Dickinson, 1964, Domsch et al., 2007.
Notes: Judging from our phylogenetic results, the species of Wardomyces do not form a monophyletic group, being scattered in three closely related lineages. The small genetic distances and inconsistent morphological differences observed between the three groups do not support the proposal of a generic status for the lineages.
To date, only Wardomyces giganteus has been described with a sexual morph, which closely resembles those observed in Microascus and Scopulariopsis. However, it can be differentiated by significantly larger ascomata, ascospores with two germ pores and dark, solitary conidia with a longitudinal germ slit, features that are never present in the other two genera sensu stricto.
Wardomyces anomalus F.T. Brooks & Hansf., Trans. Brit. mycol. Soc. 8: 137. 1923. Fig. 23.
Fig. 23.
Wardomyces anomalus (ex-epitype CBS 299.61). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–G. Conidiophores. H–I Conidia. Scale bars: D–I = 5 μm.
Material examined: Lectotype designated here: England, on meat of Oryctolagus cuniculus, 1918, J.F. Brooks and C.G. Handsford, MBT-372238 (IMI 25846). Epitype designated here: Canada, Ottawa, air cell of egg in cold storage in salt solution, 1947, W.I. Illman, MBT-202776 (culture ex-epitype CBS 299.61).
Descriptions and illustrations: Brooks and Hansford, 1923, Hennebert, 1962.
Notes: This species has been isolated from frozen stored meat and eggs, and from soil and marine environments (Dickinson 1964), and is associated with the production of antioxidant compounds (Abdel-Lateff et al. 2003). This is the type species of Wardomyces, however, no holotype material was cited in the protologue and neither is present in the different herbaria we checked (BPI, BR, CGE, E, or K) and therefore, it is presumed lost. Isotype dry material was deposited in IMI (Kew, England) and is proposed here as lectotype. Since an ex-type or ex-isotype culture does not exist, we selected an epitype culture from the authentic material referenced in Brooks & Hansford (1923) and studied here.
Wardomyces anomalus is similar to W. inflatus exhibiting hyaline, inflated to barrel shaped conidiogenous cells and similar conidial size. However, the conidia of the former species are ovoid with pointed apex and measure 4.5–8 × 3.5–6 μm, while those of W. inflatus are ellipsoidal to cylindrical with a rounded apex and measure 6–8 × 3.5–5 μm.
Wardomyces giganteus (Malloch) Sandoval-Denis, Guarro & Gené, comb. nov. MycoBank MB814579. Fig. 24
Fig. 24.
Wardomyces giganteus (ex-type CBS 746.69). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores. E–H, Polyblastic conidiogenous cells and conidia. Scale bars: D–H = 5 μm.
Basionym: Microascus giganteus Malloch, Mycologia 62: 731. 1970.
Material examined: Canada, Ontario, Simcoe Co., insect frass in dead log, 1968, D.W. Malloch (Holotype TRTC 45434; culture ex-type CBS 746.69).
Descriptions and illustrations: Malloch, 1970a, Guarro et al., 2012.
Notes: This species was originally described in Microascus because of the characters of its sexual morph. However, the morphology of the asexual morph, with polyblastic conidiogenus cells and dark, thick-walled conidia with a longitudinal germ slit does not match with the morphological characteristics of the asexual morphs of Microascus or Scopulariopsis. In addition, the morphology of its sexual morph is slightly different from Microascus; W. giganteus has large hairy ascomata and reniform ascospores with two polar germ pores. Several phylogenetic studies (Issakainen et al., 2003, Sandoval-Denis et al., 2016) point toward Wardomyces, which also agrees with the morphological evidences.
Wardomyces giganteus morphologically resembles W. humicola and W. pulvinatus, mainly in the characters of the asexual morph. However, in addition to the presence of a sexual morph in W. giganteus, this species has 1-celled, ellipsoidal and pointed conidia 6.5–14 × 3.5–5 μm, borne from hyaline, mostly solitary conidiogenous cells. In contrast, W. humicola exhibits 2-celled, ellipsoidal to navicular conidia, slightly smaller (9–12 × 2.5–5.5 μm), while the conidia of W. pulvinatus are 1-celled, smaller (5.5–10 × 3–4.5 μm), typically papillate and formed on pale brown conidiogenous cells.
Wardomyces humicola Hennebert & G.L. Barron, Canad. J. Bot. 40: 1209. 1962. Fig. 25.
Fig. 25.
Wardomyces humicola (ex-isotype CBS 369.62). A. Conidiophores. B–E. Polyblastic conidiogenous cells and conidia. Scale bars: A–E = 2 μm.
Material examined: Canada, Ontario, Guelph, Ontario Agricultural College, soil in tropical greenhouse, 1961, G.L. Barron (Holotype DAOM 75655; culture ex-isotype CBS 369.62).
Description and illustrations: Hennebert (1962).
Notes: This fungus was isolated from soil in Africa, Asia, Europe and North America (Hennebert, 1962, Domsch et al., 2007). It is phylogenetically and morphologically similar to W. pulvinatus; both species producing penicillate conidiophores and pointed conidia. However, the conidia of W. humicola are 2-celled, measuring 9–12 × 2.5–5.5 μm and are formed on hyaline, barrel-shaped conidiogenous cells, while those of W. pulvinatus are 1-celled, 5.5–10 × 3–4.5 μm, and are formed on pale brown conidiogenous cells.
Wardomyces inflatus (Marchal) Hennebert, Trans. Brit. mycol. Soc. 51: 755. 1968. Fig. 26.
Fig. 26.
Wardomyces inflatus (ex-neotype CBS 367.62). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–F. Conidiophores. G–H, Polyblastic conidiogenous cells. I–K. conidia. Scale bars: D–K = 5 μm.
Basionym: Trichosporum inflatum Marchal, Champ. copr. Belg. 7: 142. 1896.
Synonym: Wardomyces hughesii Hennebert, Canad. J. Bot. 40: 1207. 1962.
Material examined: Belgium, Heverlee, greenhouse soil under Lycopersicon esculentum, 1959, G.L. Hennebert & E. Delvaux (culture ex-neotype CBS 367.62). Canada, Quebec, Gatineau County, Ste Cécile de Masham, from decayed wood, 1960, G.L. Hennebert (culture ex-isotype of Wardomyces hughesii CBS 216.61).
Descriptions and illustrations: Hennebert, 1962, Hennebert, 1968.
Notes: Hennebert (1968) discussed the morphological similarity between W. hughesii and Trichosporum inflatum and, despite some morphological discrepancies, they were considered as conspecific. Our molecular results confirm this synonymy.
The morphological features of the conidiogenous cells (markedly constricted at the septum) and conidia (ellipsoidal to cylindrical with rounded apices, 6–8 × 3.5–5 μm) in W. inflatus clearly differentiate this species from the other members of the genus. Wardomyces anomalus, its closest phylogenetic and morphological relative, has barrel-shaped conidiogenous cells and somewhat smaller (4.5–8 × 3.5–6 μm), ovoid and pointed conidia.
Wardomyces ovalis W. Gams, Trans. Brit. mycol. Soc. 51: 798. 1968. Fig. 27.
Fig. 27.
Wardomyces ovalis (ex-type CBS 234.66). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–G. Conidiophores, polyblastic conidiogenous cells and conidia. H–I, Annellidic conidiogenous cells and conidia. Scale bars: D–I = 5 μm.
Synonym: Hennebertia ovalis (W. Gams) M. Morelet, Ann. Soc. Sci. Nat. Archéol. Toulon Var 21: 105. 1969.
Material examined: Germany, Schleswig-Holstein, Kiel-Kitzeberg, wheat-field soil, 1963, W. Gams (culture ex-type CBS 234.66).
Description and illustrations: Gams (1968).
Notes: Wardomyces ovalis resembles W. anomalus and W. inflatus. However, it is unique in the genus by the production of additional scopulariopsis-like conidiation, which is characterised by hyaline to subhyaline, 1-celled, smooth-walled conidia (5.5–10 × 3.5–6 μm) from hyaline, cylindrical annellides (6–10 × 2–4 μm).
Wardomyces pulvinatus (Marchal) C.H. Dickinson, Trans. Brit. mycol. Soc. 49: 521. 1966. Fig. 28.
Fig. 28.
Wardomyces pulvinatus (ex-type CBS 112.65). A–C. Colonies on PCA, OA and PDA respectively, after 14 d at 25 °C. D–E. Conidiophores. F–G, Conidiogenous cells. H–I. Conidia. Scale bars: D–I = 5 μm.
Basionym: Echinobotryum pulvinatum Marchal, Bull. Soc. Roy. Bot. Belgique 34: 139. 1895.
Synonym: Wardomyces papillatus C.H. Dickinson, Trans. Brit. mycol. Soc. 47: 321. 1964.
Material examined: England, Cheshire, Parkgate, salt-marsh mud under Halimione portulacoides, 1962, C.H. Dickinson (culture ex-isotype CBS 112.65).
Descriptions and illustrations: Dickinson, 1964, Dickinson, 1966, Ellis, 1971.
Notes: This species has been isolated from soil and on decaying leaves of Pandanus tectorius (Dickinson, 1966, Whitton et al., 2012).
Wardomyces pulvinatus strongly resembles W. humicola, both having navicular and pointed solitary conidia on barrel-shaped conidiogenous cells and mostly penicillately branched conidiophores. However, the conidia of W. pulvinatus are 5.5–10 × 3–4.5 μm, 1-celled, typically papillate at the apex and commonly secede, carrying a portion of the conidiogenous cell. In addition, that species is the only one in the genus forming sporodochia in culture. In contrast, the conidia of W. humicola are slightly larger (9–12 × 2.5–5.5 μm), non-papillate and 2-celled.
Wardomycopsis Udagawa & Furuya, Mycotaxon 7: 92. 1978.
Type species: Wardomycopsis inopinata Udagawa & Furuya.
Descriptions and illustrations: Barron, 1966, Udagawa and Furuya, 1978.
Notes: Wardomycopsis closely resembles Wardomyces, especially in the early stages of conidiation, both genera forming darkly pigmented conidia with a single longitudinal germ slit (Barron 1966). However, the conidiogenesis and arrangement of conidia in Wardomycopsis are more similar to those of Scopulariopsis and Fairmania species (i.e., formation of basipetal conidial chains on annellidic conidiogenous cells) rather than to Wardomyces species (i.e., formation of solitary conidia on polyblastic conidiogenous cells). The conidia of Scopulariopsis spp. are arranged in long chains and lack germ slits, while those of Fairmania present numerous (1–5) longitudinal striations.
Wardomycopsis humicola (G.L. Barron) Udagawa & Furuya, Mycotaxon 7: 96. 1978. Fig. 29.
Fig. 29.
Wardomycopsis humicola (ex-isotype CBS 487.66). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–G. Conidiophores, conidiogenous cells and conidia. Scale bars: D–G = 5 μm.
Basionym: Scopulariopsis humicola G.L. Barron, Antonie van Leeuwenhoek 32: 294. 1966.
Material examined: Canada, Ontario, Guelph, from soil, 1964, G.L. Barron (Holotype OAC 10260; culture ex-isotype CBS 487.66). Spain, Girona, Pals, from sediments of Ter River, 1991, C. Ulfig & J. Gené (FMR 3993); Reus, Institut Salvador Vilaseca, from garden soil, 2014, M. Repollés & J. Gené (FMR 13592).
Description and illustrations: Barron (1966).
Notes: Wardomycopsis humicola is a soil-borne, seldom observed species. It is phylogenetically close to Ws. inopinata, but differs in its pale brown, ovate to cylindrical conidia (4–5 × 2.5–3 μm) and absence of sexual morph. By contrast, Ws. inopinata has olivaceous brown, globose to subglobose conidia (4–5.5 × 4–5.5 μm) and can produce ascomata in culture (Udagawa & Furuya 1978).
Wardomycopsis inopinata Udagawa & Furuya, Mycotaxon 7: 92. 1978. Fig. 30
Fig. 30.
Wardomycopsis inopinata (FMR 10305). A–C. Colonies on PCA, OA and PDA, respectively, after 14 d at 25 °C. D–H. Conidiogenous cells. I–J. Conidia. Scale bars: D–J = 5 μm.
Synonym: Microascus inopinatus Udagawa & Furuya, Mycotaxon 7: 91. 1978.
Material examined: Myanmar, from soil, 2008, C. Hartung (FMR 10305); from soil, 2008, C. Hartung (FMR 10306).
Descriptions and illustrations: Udagawa & Furuya (1978).
Notes: This is the only species of the genus for which a sexual morph has been described. It is characterised by slowly forming ostiolate ascomata (up to 350 μm diam) and straw-coloured, reniform to triangular ascospores (3–3.5 × 2.5–3 μm) with a single germ pore. Nonetheless, the sexual morph was not observed in either of two isolates studied here. Although the holotype (NHL 2767, preserved at the National Institute of Hygienic Sciences, Tokyo, Japan) or ex-type cultures of Ws. inopinata are unavailable for comparison, the morphology of the asexual morph of the soil isolates investigated from Myanmar agrees with that of the protologue of Ws. inopinata, which was based on a soil isolate from Thailand (Udagawa & Furuya 1978). The phylogenetic analysis confirmed this taxon as different from the previous three species accepted in the genus. Wardomycopsis inopinata is characterised by globose to subglobose, olivaceous brown conidia, measuring 4–5.5 × 4–5.5 μm, on flask-shaped or cylindrical, 2.5–3 μm wide annellides; Ws. humicola produces narrower ovoid to cylindrical, smoky brown conidia (4–5 × 2.5–3 μm) on barrel-shaped annellides (1.5–2.5 μm wide); and Ws. litoralis has obovoid to broadly ellipsoidal, dark olive conidia (5–7 × 3–4.5 μm) and wider ampulliform annellides (3.5–5 μm wide).
Wardomycopsis litoralis Silvera et al., Mycotaxon 105: 197. 2008. Fig. 31.
Fig. 31.
Wardomycopsis litoralis (ex-type CBS 119740). A. Conidiophores. B–C. Conidiogenous cells. D–E. Conidia. Scale bars: A–E = 5 μm.
Material examined: Spain, Vinarós, Castelló, from soil, 2004, A. Stchigel (Holotype IMI 394093; culture ex-type CBS 119740 = IMI 394093 = FMR 8876).
Description and illustrations: Silvera-Simón et al. (2008).
Notes: Wardomycopsis litoralis was originally described on the basis of morphological features and ITS sequence analysis (Silvera-Simón et al. 2008). Our polyphasic approach confirms this taxon as a distinct phylogenetic species of Wardomycopsis, being characterised by dark olive brown, obovoid to broadly ellipsoidal and smooth, short-catenate conidia (5–7 × 3–4.5 μm) borne on hyaline to subhyaline ampulliform annellides (5.5–7.5 × 3.5–5 μm).
Discussion
This work adds relevant data to build a more natural taxonomy of the Microascales, and particularly of the Microascaceae. Previous studies have reviewed the molecular phylogenetic relationships among members of this family (Lackner and Hoog, 2011, Lackner et al., 2014, Sandoval-Denis et al., 2016), providing a broad phylogenetic backbone through a molecular overview of this group of fungi. It was demonstrated that this family shows an intricate phylogenetic structure composed of several genera that share similar ecological and morphological features (Lackner and Hoog, 2011, Lackner et al., 2014, Jagielski et al., 2016, Sandoval-Denis et al., 2016). Due to the lack of DNA sequences in public databases and the paucity of phylogenetic studies, several genera of the family, particularly those with synnematous conidiophores or conidia with germ slits were still of uncertain affinities. We studied a large set of isolates, including all the available living type material, of the synnematous genera Cephalotrichum, Doratomyces, Trichurus, their closest phylogenetic relatives Wardomyces and Wardomycopsis, and several related taxa of uncertain taxonomic position.
The morphology of the sexual morphs is very homogeneous in Microascaceae and particularly among Acaulium, Fairmania, Fuscoannellis, Microascus, Pithoascus, Pseudoscopulariopsis, Scopulariopsis, Wardomyces and Wardomycopsis; this explains the former placement of most of these genera as synonyms of Microascus (Morton and Smith, 1963, Guarro et al., 2012). However, our current and recent phylogenetic results (Sandoval-Denis et al., 2016, Jagielski et al., 2016) showed the above-mentioned genera to comprise distinct lineages. Their genetic diversity correlates with subtle morphological differences, such as the presence or absence of hyaline or pigmented annellidic conidiogenous cells, unicellular or septate annelloconidia and/or solitary conidia with or without germ slits (Table 2).
Interestingly, our study revealed that the genus Wardomyces is paraphyletic. Its species are distributed in three closely related lineages, with two species each. The morphological differences between these species (i.e., presence/absence of annelloconidia and/or septate solitary conidia) have previously led to the proposal to segregate Wardomyces into different genera (Morelet 1969). However, we could demonstrate that these morphological features are not constant and thus we preferred to maintain Wardomyces s. lat. until more taxa can be investigated and the morphological evidence is properly understood.
Another controversial issue has been the current status of the genus Gamsia, which has been considered a synonym of Wardomyces by most authors (Domsch et al., 2007, Seifert et al., 2011, Whitton et al., 2012). However, our results demonstrated that Gamsia constitutes a genetically distinct lineage basal to Wardomyces s. lat., being morphologically distinguished by the complexity of its conidiophores.
The genera Acaulium and Fairmania, with three and one species, respectively, have been reintroduced in this study. The phylogenetic data provided here agree with significant morphological differences for maintaining Acaulium and Fairmania as different from Microascus and Scopulariopsis, respectively, from which both genera had been previously considered synonyms.
The decision about the most appropriate name for the synnematous genera Cephalotrichum and Doratomyces, considered synonyms by numerous authors, has been a matter of discussion for a long time (Hughes, 1958, Morton and Smith, 1963, Abbott, 2000, Domsch et al., 2007), while Trichurus has been until recently considered as a different genus (Domsch et al. 2007). However, phylogenetic inference resolved a lineage comprising the Cephalotrichum lectotype suggested by Hughes (1958), and followed by subsequent authors (Abbott, 2000, Seifert et al., 2011, Beer et al., 2013). It also demonstrated that Trichurus species belong to the same lineage hence being congeneric with Cephalotrichum, as it was suggested by several authors based on morphological criteria (Hasselbring, 1896, Abbott, 2000). Our phylogenetic results confirm most of the species synonymies previously proposed by Abbott (2000) and also corroborate the chosen epitypes for several taxa.
Although Cephalotrichum has not been regarded as a human pathogen, several of the isolates included in this study were from clinical origin, particularly those belonging to C. asperulum and C. gorgonifer, mostly isolated from respiratory specimens. However, given the lack of clinical data concerning such isolates, no information on the actual pathogenic role of these isolates can be provided. Given the origin of the isolates and the common airborne dispersal method of these fungi, it is most likely that they were in fact colonisers or mere sample contaminants.
It is important to highlight the large number of reported taxa that could not be studied because of the lack of living cultures and consequently considered uncertain species. Given the usefulness and importance of combining morphological data with molecular phylogenetic studies to adhere to the requirements of the ICN (McNeill et al. 2012), it is crucial for the progress of science to encourage all authors of fungal names to deposit live material in international culture collections for future studies.
Key to taxa included in this study
-
1a
Synnemata present in culture ................. Cephalotrichum
-
1b
Synnemata usually lacking ................................................. 2
-
2a
Conidia hyaline to brown, guttulate, smooth, without slits or striations ....................................................... Acaulium
-
2b
Conidia pale to dark brown, with longitudinal slits or striations ........................................................................... 3
-
3a
Conidia with a longitudinal germ slit present ................... 4
-
3b
Conidia with 1–5 longitudinal striations .............................................................................. Fairmania singularis
-
4a
Conidia of one type, dark, with a longitudinal germ slit, formed on annellidic conidiogenous cells and arranged in short chains ........................................... Wardomycopsis
-
4b
Conidia of two types can be present, i) dark, with a longitudinal germ slit arising solitary and forming groups on polyblastic conidiogenous cells, ii) hyaline, arranged in chains on annellidic conidiogenous cells ...................................................................... 5
-
5a
Conidiophores undifferentiated; dark conidia forming dense clusters on the top of the conidiogenous cells ............................................................................... Gamsia
-
5b
Conidiophores well differentiated, branched; dark conidia borne apically and laterally in groups of 2–3 on the top of the conidiogenous cells ...................................................................................................................... Wardomyces
Key to Acaulium species
-
1a
Conidia hyaline, obovate, cylindrical or clavate .............. 2
-
1b
Conidia pale brown to brown, obovate ....................................................................................... A. caviariforme
-
2a
Sexual morph absent; conidia obovate up to 12 μm long .................................................................. A. acremonium
-
2b
Sexual morph present; conidia cylindrical to clavate up to 8 μm long .......................................... A. albonigrescens
Key to Cephalotrichum species
-
1a
Setae present on the upper part of the synnemata ....... 2
-
1b
Setae absent .................................................................. 4
-
2a
Setae straight, branched or unbranched ................................................................................... C. cylindricum
-
2b
Setae curved or flexuous .............................................. 3
-
3a
Setae flexuous, coiled, unbranched ......... C. gorgonifer
-
3b
Setae undulate and dichotomously branched .................................................................. C. dendrocephalum
-
4a
Echinobotryum-like synasexual morph present ............ 5
-
4b
Echinobotryum-like synasexual morph absent ............. 6
-
5a
Synnemata up to 3 000 μm tall; annelloconidia ellipsoidal to cylindrical, 5–9 × 4–5 μm with rounded apices ................................................................... C. stemonitis
-
5b
Synnemata up to 1 600 μm tall; annelloconidia subglobose to ellipsoidal, 6–7.5 × 2.5–4 μm with slightly pointed apices .......................................... C. hinnuleum
-
6a
Conidia distinctively rough ............................................ 7
-
6b
Conidia smooth or finely ornamented ........................... 9
-
7a
Synnemata up to 1 000 μm tall; conidia oval to ellipsoidal ................................................................... C. asperulum
-
7b
Synnemata often higher; conidia globose to ovoid ....... 8
-
8a
Conidia 4.5–7.5 μm wide, coarsely warted, grey-brown ......................................................................... C. nanum
-
8b
Conidia 3–5.5 μm wide, spirally sculpted, dark brown ............................................................ C. verrucisporum
-
9a
Conidia 3.5–5 × 2–3 μm; no growth at 35 °C .............................................................. C. microsporum
-
9b
Conidia larger; growth at 35 °C ................................. 10
-
10a
Synnemata up to 500 μm tall; conidia with rounded apex, pale to dark brown ....................................................... 11
-
10b
Synnemata higher; conidia with finely pointed apex, green-brown ............................................................... C. purpureofuscum
-
11a
Conidia asymmetrical, dark brown, 5.5–7.5 × 2.5–4 μm ................................................................... C. columnare
-
11b
Conidia symmetrical, pale brown, 6–7 × 3.5–4 μm ........................................................... C. brevistipitatum
Key to Gamsia species
-
1a
Dark conidia 4–7.5 × 3.5–5 μm with rounded apices; annelloconidia ellipsoidal, 2-celled, 8–10.5 × 3.5–5 μm .......................................................................................................................................................................................... G. aggregata
-
1b
Dark conidia 6–13 × 3.5–6.5 μm with slightly pointed apices; annelloconidia oval, 1–2-celled, 5–10.5 × 2.5–5.5 μm ................................ G. columbina
Key to Wardomyces species
-
1a
Annelloconidia present: hyaline to subhyaline, 5.5–10 × 3.5–6 μm, 1-celled; solitary dark conidia ellipsoidal, 7–11 × 4–5 μm .................................... W. ovalis
-
1b
Annelloconidia absent, only solitary dark conidia present .......................................................................................... 2
-
2a
Conidia 1-celled ............................................................... 3
-
2b
Conidia 2-celled ........................................... W. humicola
-
3a
Conidia ellipsoidal with rounded apex, 6–8 × 3.5–5 μm ......................................................................... W. inflatus
-
3b
Conidia oval to ellipsoidal with pointed apex ................... 4
-
4a
Conidia navicular, 5.5–10 × 3–4.5 μm, papillate, often seceding with a portion of the conidiogenous cell .................................................................... W. pulvinatus
-
4b
Conidia otherwise ............................................................ 5
-
5a
Conidia oval, up to 8 μm long; sexual form absent ...................................................................... W. anomalus
-
5b
Conidia ellipsoidal, up to 14 μm long; sexual morph if present with reniform ascospores, 4–5.5 × 3.5–4 μm, with two germ pores .............................................................................................................................. W. giganteus
Key to Wardomycopsis species
-
1a
Conidia globose to subglobose; sexual morph if present with reniform to triangular ascospores, 3–3.5 × 2.5–3 μm .................................................................... Ws. inopinata
-
1b
Conidia otherwise; sexual morph absent ...................... 2
-
2a
Conidia 4–5 × 2.5–3 μm, ovate to cylindrical .................................................................... Ws. humicola
-
2b
Conidia 5–7 × 3–4.5 μm, obovoidal to broadly ellipsoidal ....................................................................... Ws. litoralis
List of uncertain or excluded species
Cephalotrichum acutisporum J.J. Xu & T.Y. Zhang, Mycotaxon 117: 208. 2011.
Notes: The original description and illustration of this species morphologically resemble C. columnare. However, C. acutisporum has slightly longer synnemata (120–820 μm tall) with dark, compact stipes. Synnemata in C. columnare are up to 500 μm tall with very narrow stalks. Type material or DNA sequence data were unavailable for study.
Cephalotrichum album (Costantin) Seifert, CBS Biodiversity Series 12: 309. 2013.
Basionym: Synpenicillium album Costantin, Bull. Soc. Mycol. France. 4: 62. 1888.
Synonyms: Coremium album (Costantin) Sacc. & Traverso, Syll. fung. 19: 428. 1910.
Penicillium costantinii Bain. [as ‘costantini’], Bull. Soc. Mycol. France. 22: 205. 1906.
Scopulariopsis costantinii (Bain.) Dale, Ann. Mycol. 12: 57. 1914.
Notes: This species was recently included in Cephalotrichum as a new combination for one of the two different fungi present in the holotype of Stysanus putredinis Corda (de Beer et al. 2013).
The species is well circumscribed as “Doratomyces putredinus” in Morton and Smith (1963) and “Cephalotrichum putredinus” by Abbott (2000) for a white synnematous fungus exhibiting long chains of hyaline conidia. However, recently, Lackner & de Hoog (2011) designated an epitype for S. putredinis to fix this epithet under the new combination Parascedosporium putredinis, a fungus producing larger conidia in slimy masses, and previously regarded as conspecific with Graphium cuneiferum by other authors (Hughes, 1958, Seifert, 1985, Abbott, 2000). Thus, as indicated above, given the confuse application of the epithet “putredinis” under two different fungal concepts, de Beer et al. (2013) were forced to choose a different epithet for the white fungus described by Morton & Smith (1963) and now listed in Cephalotrichum.
Morphologically, C. album is dissimilar from other members of Cephalotrichum s. str., with white to pallid colonies lacking dematiaceous pigments and poorly developed, white synnemata. It is likely that it is related to another lineage of Microascaceae, and has considerable morphological similarity to species of Acaulium. However, no molecular sequence data is currently available for this species.
Cephalotrichum antarcticum (Speg.) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Sporocybe antarctica Speg., Bol. Acad. Nac. Ci. 11: 63. 1887.
Note: Carmarán & Novas (2003) studied the holotype of S. antarctica (LSP 33139) and considered this specimen to be a lichen.
Cephalotrichum aterrimum (Rabenh.) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Periconia aterrima Rabenh., Deutschl. Krypt.-Fl. (Leipzig) 1: 118. 1844.
Synonym: Sporocybe aterrima (Rabenh.) Sacc., Syll. Fung. 4: 607. 1886.
Notes: No type specimen was found to be examined and no comment on this taxon was included in the revision of the genus Periconia by Mason & Ellis (1953). The description of this species is too vague for a correct identification of fresh isolates.
Cephalotrichum atrofuscum (Mont.) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Stilbum atrofuscum Mont., J. Linn. Soc., Bot. 10: 358. 1868.
Synonym: Sporocybe atrofusca (Mont.) Sacc., Syll. fung 4: 605. 1886.
Notes: This species was poorly described and considered a nomen dubium by Seifert (1985) since type and isotype material preserved in K only contained sterile structures.
Cephalotrichum bulbosum (Schwein.) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Periconia bulbosa Schwein., Trans. Amer. Philos. Soc. 4: 304. 1832.
Notes: No comment on this taxon was included in the revision of Periconia by Mason & Ellis (1953) and no living material is available for a proper morphological characterisation of the species. There is not certainty about the identity of the holotype material. Two different specimens are available in PH and regarded as “probable types”.
Cephalotrichum caespitosum Demelius, Verh. Zool.-Bot. Ges. Wien 72: 99. 1923.
Notes: No type specimen was found for examination. The original description shows a fungus with catenate conidia borne on polyblastic and denticulate conidiogenous cells, which seems to indicate that it does not correspond to a Cephalotrichum species.
Cephalotrichum carneum (Richon) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Sporocybe carnea Richon, Cat. champ. Marne: no. 2088.
Note: The original description is too vague for its recognition.
Cephalotrichum castaneum (Y.L. Jiang & T.Y. Zhang) Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 224. 2011.
Basionym: Doratomyces castaneus Y.L. Jiang & T.Y. Zhang, Mycotaxon 104: 131. 2008.
Notes: The protologue describes a species of Cephalotrichum characterised by spherical to subspherical, thick-walled conidia (4–6 μm diam.). Type material was unavailable for study; however, the analysis of an ITS sequence of the ex-type (GenBank, FJ914681) had a sequence similarity of 99.2 % with C. dendrocephalum. Further studies involving more informative loci are needed to clarify the taxonomy of this species.
Cephalotrichum cellare (Peck) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Sporocybe cellaris Peck, Annual Rep. New York St. Mus. Nat. Hist. 42: 129. 1889.
Note: The description of this species is too vague for a proper identification.
Cephalotrichum clavulatum (Sacc.) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Sporocybe clavulata Sacc., Syll. Fung 4: 604. 1886.
Note: The original description of this species is too vague for a proper identification.
Cephalotrichum commune Demelius, Verh. zool.-bot. Ges. Wien 72: 98. 1923.
Notes: The original description point toward a species of Cladosporium rather than Cephalotrichum. Type material or living strains were not found for confirmation.
Cephalotrichum concentricum (Schwein.) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Coremium concentricum Schwein., Proc. Am. Phil. Soc. Phila. 4: 282. 1832.
Synonym: Sporocybe concentrica (Schwein.) Sacc., Syll. Fung. 4: 608. 1886.
Notes: Seifert & Samson (1985) studied the type material of Coremium concentricum and reported the presence of only immature or decapitated conidiomata of what may be a Mycosphaerella asexual morph, rendering this taxon to a nomen dubium.
Cephalotrichum corticale (Cooke & Peck) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Periconia corticalis Cooke & Peck, in Peck, Annual Rep. New York St. Mus. Nat. Hist. 29: 52. 1878.
Synonyms: Sporocybe corticalis (Cooke & Peck) Sacc., Syll. Fung. 4: 604. 1886.
Didymobotryum corticale (Cooke & Peck) Dearn. & House, Bull. New York State Mus. 266: 98. 1925.
Notes: No comment on this species was included in the revision of Periconia by Mason & Ellis (1953), and no living material is available for a better morphological characterisation of the species.
Cephalotrichum cylindrosporum Y.L. Zhang & T.Y. Zhang, Mycotaxon 117: 209. 2011.
Notes: The ITS sequence of the ex-type culture of this species (GenBank FJ914686) has a similarity of 100 % with the ex-epitype strain of C. stemonitis. However, these results do not match with the morphological characteristics described in the protologue of C. cylindrosporum, which has smooth conidia (5–6.2 × 2.5–32 μm) and lack of an echinobotryum-like synasexual morph. Unfortunately, the type material could not be examined after repeated request to the authors, and further studies are needed to assess the taxonomic position of this fungus. In any case, the epithet “cylindrosporum” can create confusion with the previously described species C. cylindricum.
Cephalotrichum ellipsoideum H.Q. Pan & T.Y. Zhang, Mycotaxon 117: 211. 2011.
Notes: The protologue describes a fungus morphologically similar to C. purpureofuscum. However, in C. ellipsoideum the conidia are wider and have rounded apices (6–8.5 × 3.5–6 μm versus 5–8 × 3–4.5 μm with slightly pointed apices in C. purpureofuscum). Type material was unavailable for study.
Cephalotrichum epiphyllum (Schwein.) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Periconia epiphylla Schwein., Trans. Amer. Philos. Soc. 4: 304. 1832.
Synonym: Sporocybe epiphylla (Schwein.) Sacc. Syll. fung. 4: 608. 1886.
Notes: Description too vague for a correct identification and no comment on this species was included in the revision of Periconia by Mason & Ellis (1953). A specimen deposited by Schweinitz and dated 1828 is available in PH, although it is unknown if it corresponds to the holotype for the species.
Cephalotrichum fasciculatum (Schwein.) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Periconia fasciculata Schwein., Trans. Amer. Philos. Soc. 4: 304. 1832.
Synonym: Sporocybe fasciculata (Schwein.) Sacc., Syll. Fung. 4: 607. 1886.
Notes: Description rather imprecise for a correct identification. A herbarium sheet exists in PH, although it is not known if it represents the holotype of this species.
Cephalotrichum flavovirens (Alb. & Schwein.) Nees, Syst. Pilze (Würzburg) 87: 1817.
Basionym: Periconia flavovirens Alb. & Schwein., Consp. fung. (Leipzig): 357. 1805.
Synonyms: Stilbum flavovirens (Alb. & Schwein.) Link, Sp. pl., 4: 111. 1825.
Ceratopodium flavovirens (Alb. & Schwein.) Corda, Icon. fung. (Prague) 1: 19. 1837.
Graphium flavovirens (Alb. & Schwein.) Sacc., Syll. Fung. 4: 618. 1886.
Notes: The protologue does not include any measurements to accurately identify the species. Although considered previously a Periconia species, no further data on this fungus has been found in Mason & Ellis (1953).
Cephalotrichum gracile (Schwein.) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Periconia gracilis Schwein., Trans. Amer. Philos. Soc. 4: 304. 1832.
Synonym: Sporocybe gracilis (Schwein.) Sacc. Syll. fung. 4: 608. 1886.
Notes: Description too vague for a correct identification. No data on this fungus has been found in Mason & Ellis (1953). A herbarium specimen is preserved in PH but regarded as a “possible type”.
Cephalotrichum gramineum (P. Karst.) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Sporocybe graminea P. Karst., Rev. Mycol. (Toulouse) 1888.
Notes: The description of this species is too vague for a correct identification.
Cephalotrichum inflatum Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 213. 2011.
Notes: The protologue describes a species morphologically similar to C. microsporum, but differentiated by its conidiophores composed of distinctively inflated cells. An ITS sequence of the ex-type culture available in GenBank (FJ914676) showed 99.5 % sequence similarity with the ex-epitype strain of C. microsporum. Further studies are needed to clarify the taxonomy of this taxon; however, type material was not made available for study.
Cephalotrichum lagerheimii Pat., Bull. Soc. Mycol. France. 9: 8. 1893.
Notes: The original description does not include any measurements to allow for accurate species recognition.
Cephalotrichum longicollum Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 213. 2011.
Notes: The protologue of the species refers to a fungus closely resembling C. purpureofuscum, although with slightly shorter conidia (4.5–5.5 × 2.5–4 μm) and synnemata (340–750 μm tall) versus 5–8 × 3–4.5 μm and 800–1 600 μm tall, respectively, in C. purpureofuscum. The analysis of the ITS sequence of the ex-type strain of C. longicollum (FJ914672) showed 100 % of similarity with the reference strain of C. purpureofuscum. Type material was unavailable for study.
Cephalotrichum lycopersici (Plowr.) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Sporocybe lycopersici Plowr., Fung. Dis. Tom 3: 4. 1881.
Notes: Description too vague for a correct identification.
Cephalotrichum macrocephalum Corda, Icon. fung. (Prague) 1: 19. 1837.
Synonym: Sporocybe macrocephala (Corda) Sacc., Syll. Fung. 4: 605. 1886.
Notes: The original description and illustration show a fungus similar to C. dendrocephalum, with thick stipitate synnemata with spherical heads and flexuous and branched sterile hairs, but with distinct globose and dark conidia with verrucose walls. Although the holotype (PRM 155400b) is preserved in the National Museum of the Czech Republic in Prague, it was unavailable for examination. Since no ex-type culture was deposited nor any strain of the species exists in any public culture collection for further study, the taxonomy of this fungus remains unclear.
Cephalotrichum macrosporum Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 214. 2011.
Notes: According to the protologue, this fungus closely resembles C. purpureofuscum morphologically, from which it differs by its somewhat larger conidia (5.5–12 × 2.5–4.5 μm) and smaller synnemata (200–600 μm long) versus 5–8 × 3–4.5 μm and 800–1 600 μm long, respectively, in C. purpureofuscum. The analysis of an ITS sequence of the ex-type strain of C. macrosporum (FJ914675) showed 100 % similarity with the reference strain of C. purpureofuscum. Type material was not made available for study.
Cephalotrichum maculare (Schwein.) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Periconia macularis Schwein., Trans. Amer. Philos. Soc. 4: 304. 1832.
Synonym: Sporocybe macularis (Schwein.) Sacc., Syn. Amer. bor. no. 3050.
Notes: Description too vague for a correct identification.
Cephalotrichum minimum (Fr.) Sacc., Syll. Fung. 11: 612. 1895.
Basionym: Actinocladium minimum Fr., Syst. mycol. (Lundae) 3: 353. 1832.
Notes: Description too vague for a correct identification.
Cephalotrichum monilioides (Alb. & Schwein.) Link, Sp. Pl. 6: 112. 1825.
Basionym: Isaria monilioides Alb. & Schwein., Consp. fung. (Leipzig): 362. 1805.
Synonyms: Stysanus monilioides (Alb. & Schwein.) Corda, Icon. fung. (Prague) 2: 17. 1838.
Coremium monilioides (Alb. & Schwein.) Pound & Clem., Minn. Bot. Stud. 1: 729. 1897.
Notes: The protologue lacks of microscopic details necessary for the identification of the fungus. However, the macroscopic features in the description and illustrations (i.e. white to yellowish mycelium and white synnemata) seem to match with the current concept of the genus Isaria (Hoog, 1972, Hodge et al., 2005).
Cephalotrichum oblongum J.J. Xu & T.Y. Zhang, Mycotaxon 117: 216. 2011.
Notes: The protologue describes a fungus morphologically similar to C. purpureofuscum, the most important difference being its narrower conidia (2–2.5 μm wide versus 3–4.5 μm wide in C. purpureofuscus). An ITS sequence of the ex-type strain of C. oblongum available in GenBank (FJ914667) showed 100 % similarity with the reference strain of C. purpureofuscum. Type material was unavailable for study.
Cephalotrichum ovoideum Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 217. 2011.
Notes: The protologue of C. ovoideum shows a fungus that morphologically matches C. microsporum. The most important distinctive morphologically feature of C. ovoideum being the formation of synnemata with branched stipes. However, we observed branched stipes in several Cephalotrichum species, including C. dendrocephalum, C. microsporum and C. nanum, thus not a reliable feature for species delimitation. An ITS sequence of the ex-type strain of C. ovoideum (GenBank FJ914662) showed 99.5 % similarity with the ex-epitype of C. microsporum and 100 % similarity with the ex-type strains of C. inflatum and C. robustum. Type material was not made available for study.
Cephalotrichum parasiticum (Peck) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Periconia parasitica Peck, Annual Rep. New York St. Mus. Nat. Hist. 33: 28. 1883.
Synonym: Sporocybe parasitica (Peck) Sacc., Syll. Fung. 4: 605. 1886, non Periconia parasitica Tilak, Mycopathol. Mycol. Appl. 9: 195. 1958.
Notes: The description of this species is too vague for a correct identification.
Cephalotrichum rhois (Berk. & M.A. Curtis) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Stilbum rhois Berk. & M.A. Curtis [as ‘rhoidis’], in Berkeley, Grevillea 3: 64. 1874.
Synonyms: Sporocybe rhois (Berk. & M.A. Curtis) Sacc., Syll. Fung. 4: 605. 1886.
Calicium rhois (Berk. & M.A. Curtis) Farl., in Thaxter, Mycologia 14: 103. 1922.
Notes: This is the asexual morph of the well-known fungus Amphiporthe/Cryptodiaporthe aculeans (Seifert 1985).
Cephalotrichum rigescens Link, Mag. Ges. Naturf. Freunde Berlin 3: 20. 1809.
Synonym: Sporocybe rigescens (Link) Sacc., Syll. Fung. 4: 605. 1886.
Notes: The original description is too vague for a correct identification and probably refers to a myxomycete (Domsch et al. 2007). This settled the basis for the confusion regarding Cephalotrichum and Doratomyces, as being the first species described in Cephalotrichum, C. rigescens was assumed to be the type of the genus. However, Hughes (1958) designated C. stemonitis as the lectotype of Cephalotrichum, which makes the application of the name very clear. According to Morton & Smith (1963), there is not type material available for this species.
Cephalotrichum robiniae (Schwein.) Kuntze, Revis. gen. pl. (Leipzig) 3: 453 1898.
Basionym: Periconia robiniae Schwein., Schriften Naturf. Ges. Leipzig. 1: 125. 1822.
Synonym: Sporocybe robiniae (Schwein.) Fr., Syst. mycol. (Lundae) 3: 342. 1832.
Notes: The original description of this species was very poor for a proper identification.
Cephalotrichum robustum Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 218. 2011.
Notes: Based on the protologue, C. robustum closely resembles C. microsporum. However, C. robustum exhibits slightly larger conidia (5–7 × 3–5 μm versus 3.5–5 × 2.5–3 μm in C. microsporum). An ITS sequence of the ex-type strain of C. robustum (GenBank accession number FJ914674) shows 99.5 % similarity with the ex-epitype of C. microsporum and 100 % similarity with the ex-type strains of C. inflatum and C. ovoideum. Type material was not made available.
Cephalotrichum septatum Demelius, Verh. zool.-bot. Ges. Wien 72: 102. 1923.
Notes: The original illustration shows a dematiaceous fungus with simple, septate conidiophores forming ramoconidia that resemble a species of Cladosporium rather than Cephalotrichum. No material was found in the type collection, general herbarium nor in the Petrak collection in W nor WU (pers. comm.).
Cephalotrichum spirale H.M. Liu, H.Q. Pan & T.Y. Zhang, Mycotaxon 117: 220. 2011.
Notes: The protologue describes a fungus morphologically similar to C. stemonitis, C. nanum and C. verrucisporum, the four species with ovoid to ellipsoidal verrucose conidia with somewhat overlapping sizes. An ITS sequence of the ex-type culture of C. spirale (GenBank accession number FJ914705) shows 99.5 % of similarity with the ex-type strain of C. verrucisporum. Type material was not made available.
Cephalotrichum terricola Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 221. 2011.
Notes: The protologue describes a fungus morphologically similar to C. purpureofuscum. The most important difference being is its smooth and slightly smaller conidia with rounded ends (4.5–7 × 2.5–3.5 μm versus 5–8 × 3–4.5 μm in C. purpureofuscum). An ITS sequence of the ex-type strain of C. terricola (GenBank accession number FJ914677) shows 100 % similarity with the reference strain of C. purpureofuscum. Type material was not made available for study.
Cephalotrichum tessulatum (Sacc.) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Sporocybe tessulata Sacc., Michelia 2: 299. 1881.
Notes: The original description of this species is very poor for a correct identification. However, it seems a quite unique synnematous fungus forming cubical conidia with a tiny apiculate base. The holotype (on stems of Dianthus armeria) is available in PAD, and includes a hand draw that clearly depicts the mentioned conidial shape. Because living strains are not available for a molecular characterisation, the taxonomy of this fungus remains uncertain.
Cephalotrichum truncatum (Cooke & Peck) Kuntze, Revis. gen. pl. (Leipzig) 3: 453. 1898.
Basionym: Periconia truncata Cooke & Peck, in Peck, Annual Rep. New York St. Mus. Nat. Hist. 29: 51. 1878.
Synonym: Sporocybe truncata (Cooke & Peck) Sacc., Syll. Fung. 4: 604. 1886.
Notes: Description too vague for a correct identification.
Cephalotrichum verrucipes Y.L. Jiang & T.Y. Zhang, Mycotaxon 117: 223. 2011.
Notes: Original material of this fungus was unavailable for study. The protologue suggests a species of Cephalotrichum; however, it differs considerably from members of this genus by its verruculose conidiophores with a distinctive irregular ramification pattern.
Doratomyces albus (Szilvinyi) Dominik, Ekol. Pol. 18: 595. 1970.
Basionym: Scopulariopsis alba Szilv., Zentralbl. Bakteriol. Parasitenk., Abt. 2. 103: 172. 1941.
Notes: This species is unidentifiable according to Morton & Smith (1963), but listed as a synonym of Doratomyces putredinus by Abbott (2000). See additional notes under Cephalotrichum album. The original description matches with a Cephalotrichum species, the most distinctive features are the white to yellow-white colonies. However, further studies are necessary to clarify the taxonomy of these white cephalotrichum-like fungi.
Doratomyces eichhorniae Conway & Kimbr. [as ‘eichhornius’], Mycotaxon 2: 128. 1975.
Notes: This taxon was excluded from Cephalotrichum by Abbott (2000) after examination of the ex-type culture (ATCC 28418), based on its particular macroscopic features (i.e., brown colonies with white tufts and abundant dark brown diffusible pigment) and the absence of annellidic conidiogenous cells. In addition, the original description and illustration showed conidia truncate at both ends.
Doratomyces phillipsii (Berk. & Leight.) F.J. Morton & G. Sm., Mycol. Pap. 86: 82. 1963.
Basionym: Periconia phillipsii Berk. & Leight., in Berkeley & Broome, Ann. Mag. Nat. Hist. 15: 33. 1875.
Synonyms: Sporocybe phillipsii (Berk. & Leight.) Sacc., Syll. Fung. 4: 609. 1886.
Stysanus phillipsii (Berk. & Leight.) E.W. Mason & M.B. Ellis, Mycol. Pap. 56: 40. 1953.
Cephalotrichum phillipsii (Berk. & Leight.) S. Hughes, Canad. J. Bot. 36: 744. 1958.
Leightoniomyces phillipsii (Berk. & Leight.) D. Hawksw. & B. Sutton, J. Linn. Soc., Bot. 75: 204. 1977.
Notes: This taxon became the type species of the genus Leightoniomyces, erected to accommodate algicolous/lichenicolous fungi morphologically distinct from Cephalotrichum/Doratomyces. It is recognised by the shape and scar of the conidia, which are produced solitary from annellidic conidiogenous cells and tend to become markedly verrucose at maturity (Hawksworth 1977).
Doratomyces putredinis (Corda) F.J. Morton & G. Sm., Mycol. Pap. 86: 83. 1963. (See notes in Cephalotrichum album).
Doratomyces sambuci P. Crouan & H. Crouan, Florule Finistère (Paris): 15. 1867.
Notes: Original description inadequate for species recognition (Morton & Smith 1963).
Doratomyces tenuis Corda, Icon. fung. (Prague) 1: 19. 1837.
Notes: The description of this species was incomplete for its recognition (Morton & Smith 1963).
Doratomyces viridis Corda, Weitenweber's Beitr. Nat. 1: tab. 5: 262B. 1837.
Notes: Morton & Smith (1963) examined the type specimen and concluded that it does not belong to Cephalotrichum (as Doratomyces). However, its identity was not determined.
Wardomyces moseri W. Gams, Sydowia Beih. 10: 67. 1995.
Material examined: Colombia, Department of Meta, from Mauritia minor, 1980, W. Gams (culture ex-isotype CBS 164.80).
Notes: Although this fungus forms sporodochium-like structures and its conidia are usually aggregated in slimy masses, it was originally included in Wardomyces (Gams 1995). Our analysis of the LSU and ITS sequences of the ex-type culture showed that this taxon does not belong to Wardomyces, clustering among the Xylariales, and related to members of the Amphisphaeriaceae and Clypeosphaeriaceae (data not shown).
Wardomycopsis trachycarpicola Joanne E. Taylor et al., Fungal Diversity Res. Ser. 12: 370. 2003.
Notes: According to the original description this species seems to be morphologically close to Wardomycopsis. However, while species of this genus produce catenate conidia on annellidic conidiogenous cells, Ws. trachycarpicola produces only solitary conidia. According to Silvera-Simón et al. (2008), this species is only known from the type specimen. Because living strains are not available for a molecular characterisation, the taxonomy of this fungus remains uncertain.
Acknowledgements
The authors are very grateful to the members of the following institutions: Ann Bogaerts (BR), Anton Igersheim (W), Bryn Dentinger and Lee Davies (K), Christine Bartram (CGE), Elana Benamy (PH), Jan Holec (PRM), Lisa A. Castlebury (BPI), Nicolien Sol (L), Robert Lücking (B), Rossella Marcucci (PAD) and Walter Till (WU). We also thank to Keith Seifert and an anonymous reviewer for their valuable comments and suggestions to improve the content of the manuscript. This study was supported by the Spanish Ministerio de Economía y Competitividad, grants CGL 2011-27185 and CGL2013-43789-P.
Footnotes
Peer review under responsibility of CBS-KNAW Fungal Biodiversity Centre.
References
- Abbott S.P. Department of Biological Sciences, University of Alberta; Canada: 2000. Holomorph studies of the Microascaceae. Ph.D. dissertation. [Google Scholar]
- Abdel-Lateff A., Klemke C., König G.M. Two new xanthone derivatives from the algicolous marine fungus Wardomyces anomalus. Journal of Natural Products. 2003;66:706–708. doi: 10.1021/np020518b. [DOI] [PubMed] [Google Scholar]
- Arx JA von. 3rd edn. Verlag J. Cramer; Vaduz, Liechtenstein: 1981. The genera of fungi sporulating in pure culture. [Google Scholar]
- Arx JA von, Figueras M.J., Guarro J. Sordariaceous ascomycetes without ascospore ejaculation. Beihefte zur Nova Hedwigia. 1988;94:1–104. [Google Scholar]
- Bainier G. Mycothèque de lÉcole de Pharmacie, XXI–XXIII. Bulletin de la Société Mycologique de France. 1907;23:218–241. [Google Scholar]
- Barron G.L. A new species of Scopulariopsis from soil. Antonie van Leeuwenhoek. 1966;32:293–298. doi: 10.1007/BF02097471. [DOI] [PubMed] [Google Scholar]
- Barron G.L., Cain R.F., Gilman J.C. The genus Microascus. Canadian Journal of Botany. 1961;39:1609–1631. [Google Scholar]
- Beer ZW de, Seifert K.A., Wingfield M.J. The ophiostomatoid fungi: their dual position in the Sordariomycetes. In: Seifert K.A., de Beer Z.W., Wingfield M.J., editors. The ophiostomatoid fungi: expanding frontiers. CBS-KNAW Fungal Biodiversity Centre; The Netherlands: 2013. pp. 1–19. (CBS biodiversity series 12). [Google Scholar]
- Brooks F.T., Hansford C.G. Mould growths upon cold-store meat. Transactions of the British Mycological Society. 1923;8:113–142. [Google Scholar]
- Carmarán C.C., Novas M.V. A review of Spegazzini taxa of Periconia and Sporocybe after over 115 years. Fungal Diversity. 2003;14:67–76. [Google Scholar]
- Carmichael J.W., Kendrick W., Conners I.L. The University of Alberta Press; Canada: 1980. Genera of Hyphomycetes. [Google Scholar]
- Clements F.E. Report on collections made in 1894–95. Botanical Survey of Nebraska. 1896;4:1–48. [Google Scholar]
- Crous P.W., Gams W., Stalpers J.A. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology. 2004;50:19–22. [Google Scholar]
- Curzi M. Rapporti fra i generi Microascus Zukal e Scopulariopsis Bainier. Bolletino della Stazione di Patologia Vegetale di Roma. 1931;11:55–60. [Google Scholar]
- Delacroix E.G. Quelques espèces nouvelles. Bulletin de la Société Mycologique de France. 1897;13:114–127. [Google Scholar]
- Dickinson C.H. The genus Wardomyces. Transactions of the British Mycological Society. 1964;47:321–325. [Google Scholar]
- Dickinson C.H. Wardomyces pulvinata comb. nov. Transactions of the British Mycological Society. 1966;49:521–522. [Google Scholar]
- Domsch K.H., Gams W., Anderson T.H. 2nd edn. IHW Verlag; Eching, Germany: 2007. Compendium of soil fungi. [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis M.B. Commonwealth Mycological Institute; Kew, England: 1971. Dematiaceous Hyphomycetes. [Google Scholar]
- Ellis M.B. Commonwealth Mycological Institute; Kew, England: 1976. More Dematiaceous Hyphomycetes. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fries E.M. 1832. Systema mycologicum: sistens fungorum ordines, genera et species, huc usque cognitas, quas ad normam methodi naturalis determinavit. Lundae. [Google Scholar]
- Gams W. Two new species of Wardomyces. Transactions of the British Mycological Society. 1968;51:798–802. [Google Scholar]
- Gams W. An unusual species of Wardomyces (Hyphomycetes) Beihefte zur Sydowia. 1995;10:67–72. [Google Scholar]
- Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradisar H., Kern S., Friedrich J. Keratinase of Doratomyces microsporus. Applied Microbiology and Biotechnology. 2000;53:196–200. doi: 10.1007/s002530050008. [DOI] [PubMed] [Google Scholar]
- Guarro J., Gené J., Stchigel A.M. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2012. Atlas of soil ascomycetes. (CBS biodiversity series 10). [Google Scholar]
- Hammill T.M. Transmission electron microscopy of annellides and conidiogenesis in the synnematal hyphomycete Trichurus spiralis. Canadian Journal of Botany. 1977;55:233–244. [Google Scholar]
- Hasselbring H. Comparative study of the development of Trichurus spiralis and Stysanus stemonitis. Botanical Gazette Crawfordsville. 1896;29:312–322. [Google Scholar]
- Hawksworth D.L. Three new genera of lichenicolous fungi. Botanical Journal of the Linnean Society. 1977;75:195–209. [Google Scholar]
- Hennebert G.L. Wardomyces and Asteromyces. Canadian Journal of Botany. 1962;40:1203–1216. [Google Scholar]
- Hennebert G.L. Echinobotryum, Wardomyces and Mammaria. Transactions of the British Mycological Society. 1968;51:749–762. [Google Scholar]
- Hodge K.T., Gams W., Samson R.A. Lectotypification and status of Isaria Pers.: Fr. Taxon. 2005;54:5–9. [Google Scholar]
- Hoog GS de. The genera Beauveria, Isaria, Tritirachium and Acrodontium gen. nov. Studies in Mycology. 1972;1:1–41. [Google Scholar]
- Hoog GS de, Guarro J., Gené J., Figueras M.J. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2011. Atlas of clinical fungi. CD-ROM version 3.1. [Google Scholar]
- Hublin A., Gradisar H., Friedrich J. Stability and stabilisation of Doratomyces microsporus keratinase. Biocatalysis and Biotransformation. 2002;20:329–336. [Google Scholar]
- Huelsenbeck J.P., Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Hughes S.J. Revisiones hyphomycetum aliquot cum appendice de nominibus rejiciendis. Canadian Journal of Botany. 1958;36:727–836. [Google Scholar]
- Issakainen J., Jalava J., Hyvönen J. Relationships of Scopulariopsis based on LSU rDNA sequences. Medical Mycology. 2003;41:31–42. doi: 10.1080/mmy.41.1.31.42. [DOI] [PubMed] [Google Scholar]
- Jagielski T., Sandoval-Denis M., Yu J. Molecular taxonomy of scopulariopsis-like fungi with description of new clinical and environmental species. Fungal Biology. 2016;120:586–602. doi: 10.1016/j.funbio.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Jiang Y.L., Xu J.J., Wu Y.M. Studies on Cephalotrichum from soils in China — twelve new species and two new combinations. Mycotaxon. 2011;117:207–225. [Google Scholar]
- Jiang Y.L., Zhang T.Y. Two new species of Doratomyces from soil. Mycotaxon. 2008;104:131–134. [Google Scholar]
- Kirk P.M., Stalpers J.A., Braun U. A without-prejudice list of generic names of fungi for protection under the International Code of Nomenclature for algae, fungi, and plants. IMA Fungus. 2013;4:381–443. doi: 10.5598/imafungus.2013.04.02.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornerup A., Wanscher J.H. 3rd edn. Methuen; London, England: 1978. Methuen handbook of colour. [Google Scholar]
- Lackner M., Hoog GS de. Parascedosporium and its relatives: phylogeny and ecological trends. IMA Fungus. 2011;21:39–48. doi: 10.5598/imafungus.2011.02.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner M., Hoog GS de, Yang L. Proposed nomenclature for Pseudallescheria, Scedosporium and related genera. Fungal Diversity. 2014;67:1–10. [Google Scholar]
- Link H.F. Observationes in ordines plantarum naturales. Dissertatio I. Magazin der Gesellschaft Naturforschender Freunde Berlin. 1809;3:3–42. [Google Scholar]
- Lumley T.C., Abbott S.P., Currah R.S. Microscopic ascomycetes isolated from rotting wood in the boreal forest. Mycotaxon. 2000;74:395–414. [Google Scholar]
- Malloch D. New concepts in the Microascaceae illustrated by two new species. Mycologia. 1970;62:727–740. [Google Scholar]
- Malloch D. Wardomyces aggregatus sp. nov. and its possible relationship to Gymnodochium fimicolum. Canadian Journal of Botany. 1970;48:883–885. [Google Scholar]
- Malloch D., Cain R.F. The genus Kernia. Canadian Journal of Botany. 1971;49:855–867. [Google Scholar]
- Malloch D., Hubart J.M. An undescribed species of Microascus from the Cave of Ramioul. Canadian Journal of Botany. 1987;65:2384–2388. [Google Scholar]
- Mason E.W., Ellis M.B. British species of Periconia. Mycological Papers. 1953;56:1–127. [Google Scholar]
- Mason-Gamer R., Kellogg E. Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae) Systematic Biology. 1996;45:524–545. [Google Scholar]
- McNeill J., Barrie F.F., Buck W.R., editors. International Code of Nomenclature for algae, fungi and plants (Melbourne Code) A.R.G. Ganter Verlag KG; 2012. [Regnum Vegetabile no. 154] [Google Scholar]
- Morelet M. Micromycètes du var et d'ailleurs (2me Note) Annales de la Société des Sciences Naturelles et d'Archéologie de Toulon et du Var. 1969;21:104–106. [Google Scholar]
- Morton F.J., Smith G. The genera Scopulariopsis Bainier, Microascus Zukal, and Doratomyces Corda. Mycological Papers. 1963;86:1–96. [Google Scholar]
- Nylander J.A. Evolutionary Biology Centre, Uppsala University; Sweden: 2004. MrModeltest v2. Program distributed by the author. [Google Scholar]
- Posada D., Buckley T.R. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Systematic Biology. 2004;53:793–808. doi: 10.1080/10635150490522304. [DOI] [PubMed] [Google Scholar]
- Rehner S.A., Buckley E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia. 2005;97:84–98. doi: 10.3852/mycologia.97.1.84. [DOI] [PubMed] [Google Scholar]
- Sandoval-Denis M., Gené J., Sutton D.A. Redefining Microascus, Scopulariopsis and allied genera. Persoonia. 2016;36:1–36. doi: 10.3767/003158516X688027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Denis M., Sutton D.A., Fothergill A.W. Scopulariopsis, a poorly known opportunistic fungus: spectrum of species in clinical samples and in vitro responses to antifungal drugs. Journal of Clinical Microbiology. 2013;51:3937–3943. doi: 10.1128/JCM.01927-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert K.A. A monograph of Stilbella and some allied Hyphomycetes. Studies in Mycology. 1985;27:1–235. [Google Scholar]
- Seifert K.A., Morgan-Jones G.A., Gams W., Kendrick W.B. CBS-KNAW Fungal BIodiversity Centre; Utrecht, The Netherlands: 2011. The genera of Hyphomycetes. (CBS biodiversity series 9). [Google Scholar]
- Seifert K.A., Samson R.A. The genus Coremium and the synnematous Penicillia. In: Samson R.A., Pitt J.I., editors. Advances in Peniciliium and Aspergillus systematics. Plenum Press; New York: 1985. pp. 143–154. [Google Scholar]
- Silvera-Simón C., Gené J., Cano J. Wardomycopsis litoralis, a new soil-borne hyphomycete from Spain. Mycotaxon. 2008;105:195–202. [Google Scholar]
- Sopp O.J. Monographie der Pilzgruppe Penicillium mit besonderer Berücksichtigung der in Norwegen gefundenen Arten. Skrifter udgivne af Videnskabs-Selskabet i Christiania. Mathematisk-Naturvidenskabelig Klasse. 1912;11:1–208. [Google Scholar]
- Sturm J. Deutschlands Flora, Abt. III. Die Pilze Deutschlands. 1829;2:1–136. [Google Scholar]
- Sugiyama J., Kawasaki Y., Kurata H. Wardomyces simplex, a new hyphomycete from milled rice. The Botanical Magazine, Tokyo. 1968;81:243–250. [Google Scholar]
- Sugiyama J., Kawasaki Y., Kurata H. Wardomyces simplex (Hyphomycetes) and its annellospores. The Botanical Magazine, Tokyo. 1969;82:353–358. [Google Scholar]
- Swart H.J. A study of the production of coremia in three species of the genus Trichurus. Antonie van Leeuwenhoek. 1964;30:257–260. doi: 10.1007/BF02046731. [DOI] [PubMed] [Google Scholar]
- Swart H.J. Doratomyces columnaris sp. nov. Acta Botanica Neerlandica. 1967;15:521–523. [Google Scholar]
- Swift M.E. Contributions to a mycological flora of local soils. Mycologia. 1929;21:204–221. [Google Scholar]
- Tamura K., Stecher G., Peterson D. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa S. Microascaceae in Japan. The Journal of General and Applied Microbiology. 1963;9:137–148. [Google Scholar]
- Udagawa S., Awao T. Notes on some Japanese Ascomycetes. VIII. Transactions of the Mycological Society of Japan. 1969;10:1–8. [Google Scholar]
- Udagawa S., Furuya K. A new species of Microascus and its peculiar conidial state. Mycotaxon. 1978;7:91–96. [Google Scholar]
- Udagawa S., Horie Y., Abdullah S. Trichurus dendrocephalus sp. nov., from Iraqui soil. Mycotaxon. 1985;23:253–259. [Google Scholar]
- Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R., Sun B.L. Ancient and recent patterns of geographic speciation in the oyster mushroom Pleurotus revealed by phylogenetic analysis of ribosomal DNA sequences. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:4599–4603. doi: 10.1073/pnas.91.10.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T.J., Bruns T., Lee S. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., editors. PCR protocols: a guide to methods and applications. Academic Press; New York, USA: 1990. pp. 315–322. [Google Scholar]
- Whitton S.R., McKenzie E.H.C., Hyde K.D. Springer Science & Business Media; The Netherlands: 2012. Fungi associated with Pandanaceae. (Fungal diversity research series). [Google Scholar]
- Wiens J.J. Testing phylogenetic methods with tree congruence: phylogenetic analysis of polymorphic morphological characters in phrynosomatid lizards. Systematic Biology. 1998;47:427–444. [Google Scholar]
- Wright J.E., Marchand S. Micoflora del suelo de la Argentina. III. Dos interesantes géneros sinemáticos: Trichurus y Doratomyces. Boletín de la Sociedad Argentina de Botánica. 1972;14:305–310. [Google Scholar]
- Zhang Y.L., Wu Y.M., Zhang T.Y. Notes on soil dematiaceous hyphomycetes from Hainan Province China I. Mycosystema. 2014;33:945–953. [Google Scholar]