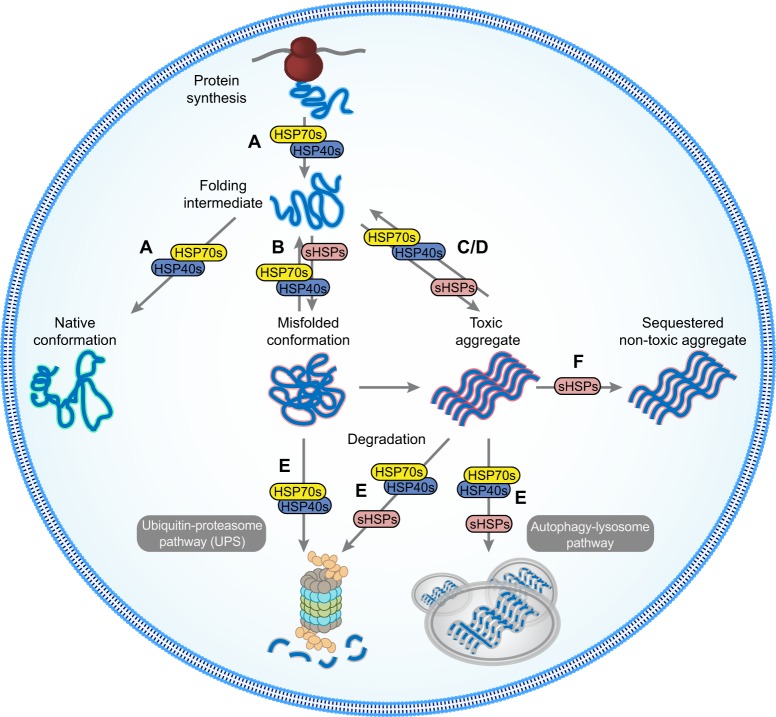
Fig. 4.
Key chaperome modifier activities in misfolding-disease progression. HSP70s and their HSP40 co-chaperones function in a variety of basic cellular quality control processes. Distinct combinations of HSP70s and HSP40s facilitate folding (A), refolding of misfolded proteins (B), preventing aggregation (C) or promoting disaggregation (D), and degradation of misfolded proteins (E). Recent therapeutic strategies have focused on partitioning HSP70 activity towards prevention of aggregation (C), disaggregation (D) and degradation (E) to maintain the integrity of the proteome. sHSPs also manage misfolded proteins (B-E) and also act as cellular shields, interacting with misfolded or aggregated proteins to prevent aberrant interaction with cellular proteins (F). In this capacity, sHSPs can interact with disease protein aggregates, sequestering these toxic aggregates and protecting cells.