Figure 7.
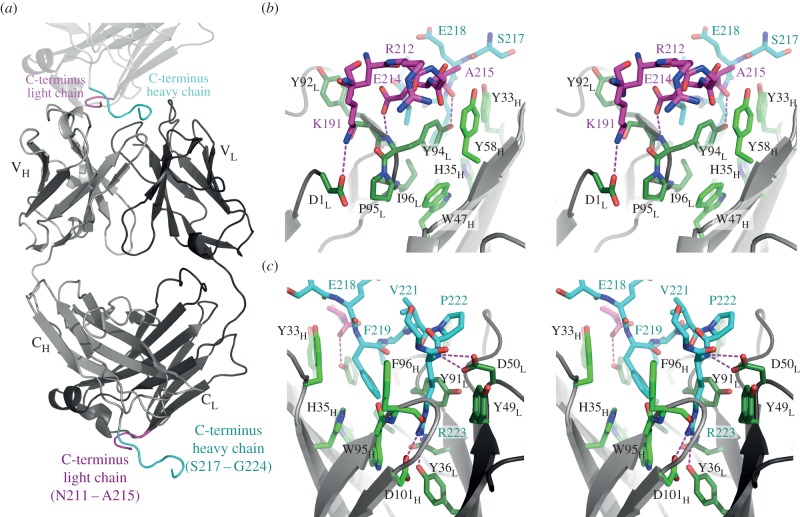
The C-termini of the Fab light and heavy chain potentially mimic the interaction of sclerostin with the Fab AbD09097. (a) Crystal-lattice contacts (the second Fab molecule is indicated in light grey, being stacked on top of the other Fab molecule) between symmetry-related Fab molecules result in the interaction of the C-terminus of the light chain (aa N211–A215 and K191) with the CDRs L1, L3, H2 and H3 of the antibody. Similarly, the C-terminus of the heavy chain comprising mainly the residues of the thrombin cleavage site for the removal of the Myc and hexahistidine-tag (aa S217–G224) interacts with the deep pocket formed by the CDRs L1, L2, L3, H1 and H3. (b) Magnification (stereo view) of the interaction of the C-terminus of the light chain (carbon atoms coloured in magenta) with the binding site of the Fab. Selected hydrogen bonds are marked as magenta stippled lines. Residues of the light chain are coloured in dark green and labels contain a subscript L. Residues of the heavy chain are coloured in green and labels contain a subscript H. Numbering of the residues located in the CDRs follows the rules of Kabat. (c) As in (b) but rotated around the y-axis by 180° to show the interaction of the C-terminus of the heavy chain (carbon atoms coloured in cyan) with the antigen-binding site of the Fab. Two amino acid residues of the thrombin cleavage site, F219 and R223, deeply penetrate into the pocket. Arginine 223 forms multiple hydrogen bonds emanating from its main and side chain atoms to residues Y36 and D50 of the light chain and F96 and D101 of the heavy chain.