Summary
For many bacteria, motility is essential for survival, growth, virulence, biofilm formation and intra/interspecies interactions. Since natural environments differ, bacteria have evolved remarkable motility systems to adapt, including swimming in aqueous media, and swarming, twitching and gliding on solid and semi-solid surfaces. Although tremendous advances have been achieved in understanding swimming and swarming motilities powered by flagella, and twitching motility powered by Type IV pili, little is known about gliding motility. Bacterial gliders are a heterogeneous group containing diverse bacteria that utilize surface motilities that do not depend on traditional flagella or pili, but are powered by mechanisms that are less well understood. Recently, advances in our understanding of the molecular machineries for several gliding bacteria revealed the roles of modified ion channels, secretion systems and unique machinery for surface movements. These novel mechanisms provide rich source materials for studying the function and evolution of complex microbial nano-machines. In this review, we summarize recent findings made on the gliding mechanisms of the myxobacteria, flavobacteria and mycoplasmas.
Graphical abstract
Surfaces are natural habitats for many bacterial species. Some bacteria glide on surfaces without the aid of traditional flagella or pili. The mechanisms of gliding are not well understood. Recent advances revealed that gliding in different bacteria involves diverse motors and a broad spectrum of mechanisms. This review summarizes recent findings on the gliding mechanisms of the myxobacteria, flavobacteria and mycoplasmas.
Introduction
Surfaces are natural habitats for many bacterial species. Surfaces enable bacteria to cooperate with kin, compete with other microorganisms, construct complex biofilms, and interface with humans and other eukaryotic hosts (Persat et al., 2015). To move actively over surfaces, bacteria employ several behaviors including swarming, twitching, gliding, and host tissue interactions (Henrichsen, 1972, Dubreuil et al., 2002). Swarming occurs on soft and moist surfaces and cells are propelled by the rotation of flagella (Kearns, 2010). Twitching motility also functions on soft and moist surfaces and is powered by the extension of type IV pili; pilus retraction can subsequently pull cells forward (Li et al., 2003, Maier et al., 2002, Skerker & Berg, 2001, Chang et al., 2016). Gliding motility, on the other hand, usually functions on firmer and drier surfaces. Because the motors for gliding were unknown and gliding bacteria have no obvious external structures associated with motility, gliding was traditionally defined by the motors that were not used (flagella and pili) rather than by the motors that were used. This unfortunate definition caused the presumption that the mechanism for gliding in diverse bacteria is likely to be similar.
In the past decade, genomic information has expedited the discovery of motors and motility-related genes in many species. Additionally, the application of advanced electron microscopy (EM) techniques such as cryo-electron tomography (cryo-ET) has enabled the visualization of novel motility-related protein complexes. Furthermore, super-resolution fluorescence microscopy has enabled us to monitor the dynamics of motility-related proteins in live cells. These results revealed that gliding in different bacteria involves diverse motors and a broad spectrum of mechanisms. The aim of this review is to summarize recent findings on gliding in the myxobacteria, flavobacteria and mycoplasmas. Although our knowledge is still limited with many pieces missing, we can already take a peek at the beautiful dynamics of these molecular machineries.
Myxobacterial gliding is powered by modified flagella stator complexes that move rapidly within the membrane
Myxobacteria are Gram-negative δ-proteobacteria. In the order of Myxococcales, most species are rod-shaped soil bacteria that feature surface movements and fruiting body formation. Myxococcus xanthus, the best studied myxobacterium, is a model organism for studying surface motility, social behaviors, biofilm formation, and interspecies interaction such as predation (Zusman et al., 2007, Keane & Berleman, 2016). M. xanthus lacks flagella and is unable to swim in liquid culture. Instead, it employs two distinct mechanisms to move on surfaces: gliding and twitching (Nan & Zusman, 2011). Twitching motility in M. xanthus is based on the extension and retraction of type IV pili, similar to that of Pseudomonas and Neisseria (Wu & Kaiser, 1995, Chang et al., 2016). By contrast, gliding motility in M. xanthus appears to be unlike other characterized prokaryotic motility systems. Despite the identification of dozens of gliding-related genes (Hodgkin, 1979, Youderian et al., 2003), the search for the gliding motors lasted for decades. In 2011, two groups reported that a proton channel formed by three proteins AglR, AglQ and AglS is essential for gliding. Importantly, this proton channel/motor complex is homologous to the Escherichia coli flagella stator complex MotAB (AglR is a MotA homologue while AglQ and AglS are MotB homologues), suggesting that gliding is powered by proton motive force (PMF) (Nan et al., 2011, Sun et al., 2011). This hypothesis was confirmed by the isolation of a point mutation in the putative proton-binding site in AglQ that completely abolished gliding (Sun et al., 2011).
Since M. xanthus gliding does not depend on visible surface appendages, it is still an open question as to how motor proteins in the inner membrane can propagate mechanical force to the cell surface and propel the movement of the cell body. An important clue to this enigma came from a comparison of the MotB homologues from M. xanthus with the E. coli MotB: both AglQ and AglS from M. xanthus lack the C-terminal peptidoglycan attachment motif. Since the M. xanthus AglRQS stator complex, unlike its E. coli homologue, was untethered, it could hypothetically be free to move within the membrane. This possibility was confirmed by direct observation of fluorescently tagged AglR and AglQ using super-resolution microscopy (Nan et al., 2013):
Super-resolution microscopy techniques, such as the single-particle tracking photoactivated localization microscopy (sptPALM), are capable of pinpointing the location of individual protein particles with sub-diffraction resolution (<100 nm), and to resolve real time molecular dynamics in live cells (Manley et al., 2008). To study the mechanism by which the AglRQS channel powers gliding, AglR and AglQ were labeled with photoactivatable fluorophores and their molecular dynamics studied at 100-ms time resolution using sptPALM (Nan et al., 2015, Nan et al., 2013). These studies found that single AglRQS channels move in helical trajectories at up to 3-5 μm/s, indicating that rather than being restricted in the membrane, the AglRQS channel functions as the core component in the gliding motor complex. Collectively, the motion of hundreds of motor complexes can appear as rotating helices inside each cell (Nan et al., 2015, Nan et al., 2013). Careful analysis of the molecular behavior of the AglR protein revealed a striking phenomenon: on a firm surface, the fast-moving motor complexes tend to slow down and accumulate at a few “traffic jam” sites on the ventral sides of cells, where the cells contact the gliding surface (Nan et al., 2013). These sites are dynamic as motor complexes continuously enter and leave the clusters. The clusters distribute evenly along the cell body due to helix periodicity and appear to remain near stationary as cells move forward (Nan et al., 2011, Nan et al., 2013) (Fig. 1A).
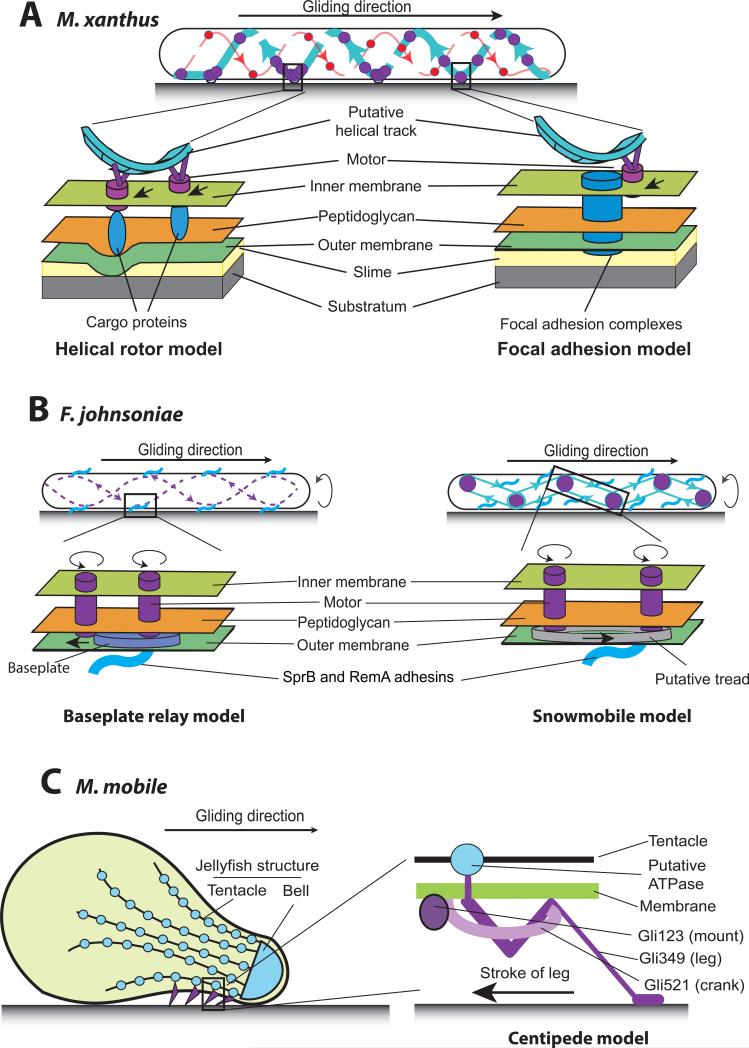
Fig. 1.
Models for gliding motility in M. xanthus (A), F. johnsoniae (B) and M. mobile (C). (A) Gliding in M. xanthus is powered by MotAB homologues that move along helical tracks in the inner membrane. Two models propose different mechanisms by which cells transform the proton motive force from the inner membrane into a mechanical force on the cell surface. The helical rotor model proposes that due to the increased resistance, the velocity of motor complexes within the membrane slows down at the sites where cells contact the surface. The slowed motor complexes accumulate in dynamic “traffic jams” that deform the cell envelope, push against the surface, and generate a backward surface wave that propels cells forward. By contrast, the focal adhesion model proposes that focal adhesion complexes (FACs) penetrate the cell envelope and anchor cells to the gliding surface. The motor complexes propel cells forward by pushing the FACs backward. (B) Gliding in F. johnsoniae is propelled by unknown motors that transport surface adhesins. The baseplate relay model proposes that helically arranged rotary motor units transport surface adhesins on baseplates. The rotation of the motors passes the adhesins along the track. The snowmobile model predicts that motor units are connected to a belt system similar to the treads that link sprockets in a snowmobile. Thus rotation of motor units transports the adhesins along those conveyor belts. (C) The “neck” region of the M. mobile cell surface is covered by a matrix of protein “legs” that attach to a jellyfish-like cytoskeletal structure. The centipede model proposes that cells are propelled by the strokes of numerous legs, which depend on ATP hydrolysis.
These results help to explain earlier data. By standard resolution microscopy, the fluorescently-labeled proteins, AglR, AglQ and the motor-associated proteins AgmU and AglZ all appeared as blurry fluorescent patches or clusters that change shape and localization constantly. Despite their different cellular localization (AglR and AglQ in the membrane, AgmU in periplasm and AglZ in cytoplasm), when cells were moving on a solid surface all four proteins showed a common feature: they tended to aggregate into a few fluorescent spots that evenly distributed along the long cell axes. Surprisingly, when cells moved forward, these protein clusters did not move along with the cells but remained at fixed positions with respect to the substratum (Mignot et al., 2007, Nan et al., 2013, Nan et al., 2011, Nan et al., 2010, Sun et al., 2011). In other words, the cells appeared to move through these spots, a behavior similar to the eukaryotic motilities that depend on focal adhesions (Smilenov et al., 1999) (Fig. 1A). When cells were placed in a liquid broth or in 1% methylcellulose, the labeled proteins appeared to decorate a rotating helix; however, these cells could not move by gliding as they lacked a solid surface (Nan et al., 2013, Nan et al., 2011). Based on the above experimental observations, two models were proposed to interpret the aggregation of motor clusters and to explain the mechanism by which cells transform PMF from the inner membrane into mechanical forces on the cell surface.
The focal adhesion model interprets the aggregates of motor complexes as rigid focal adhesion clusters (FACs). According to this model, each locus contains multiple FACs that span the cell envelope and anchor to the substratum. The gliding motor complexes push against FACs, and thus transport these FACs linearly towards the posterior end of the cells. Since FACs and adhesins are proposed to anchor cells to the gliding surface, the backward translocation of FACs would propel cells forward (Mignot et al., 2007, Sun et al., 2011) (Fig. 1A). The exact composition of the putative FACs is still unknown. However, dozens of proteins were found to associate with the gliding complexes, including cytoplasmic, periplasmic and integral membrane proteins and lipoproteins that attach to inner and outer membrane (Luciano et al., 2011, Nan et al., 2010, Jakobczak et al., 2015, Youderian et al., 2003). A possible problem encountered by the focal adhesion model is breaching the cell wall barrier, as the FACs are proposed to repeatedly sever the rigid peptidoglycan layer in order to push the cell body forward. However, it is possible that cells have evolved a novel mechanism to circumvent the cell wall problem, which has not yet been recognized. Over 40 genes have been reported as important for gliding motility in M. xanthus, but most of these genes have functions that have not yet been determined.
The helical rotor model proposes that the seemingly stationary fluorescence spots seen in gliding cells on surfaces are actually caused by the transient accumulation of motor complexes caught in dynamic “traffic jams.” According to the model, the motor complexes and associated proteins move rapidly in a helical pathway through the membrane, temporarily slowing down when encountering resistance from the gliding substratum. Evidence for these “traffic jams” comes from the movement of motor complexes in cells placed on agar of different composition. On harder agar, clusters of motor complexes appear larger and individual motor complexes slow down significantly; however, on leaving the cluster sites, their maximal velocity is restored (Nan et al., 2013, Nan et al., 2010). The accumulated motor complexes in these traffic jam sites (and their associated proteins) are proposed to exert a force that slightly deforms the cell envelope, generating a backward surface wave as the motor complexes push backward, analogous to a crawling snail. Accordingly, these traffic jam sites would act as force generators to propel the cells forward (Nan et al., 2014, Nan & Zusman, 2011). For detailed computer simulation, see (Nan et al., 2011) (Fig. 1A). Indeed, regular spaced surface distortions were visualized using total internal reflection fluorescence microscopy (Nan et al., 2011) and scanning EM (Lunsdorf & Schairer, 2001, Pelling et al., 2005). According to biophysical modeling, this mechanism should provide enough thrust to move the cells forward while avoiding breaching the cell wall (Nan et al., 2011). It is worth noting that the helical rotor model does require adhesion between the cell surface and the gliding substratum. First, adhesive materials such as slime are required to allow the helical waves to transmit the propulsive force to the substrate (Nan et al., 2011, Nan et al., 2014). Second, according to computational modeling, a certain degree of surface adhesion is required for the maintenance of gliding direction (Balagam et al., 2014).
The even distribution of the aggregates of motor proteins and the helical motion of the motor complexes both suggest the involvement of a helical structure in the cell (Mignot et al., 2007, Nan et al., 2013). In fact, MreB, the bacterial actin homologue that has the potential to form helical filaments was found essential for gliding motility in M. xanthus (Mauriello et al., 2010, Nan et al., 2013, Nan et al., 2011, Treuner-Lange et al., 2015). The M. xanthus MreB filaments appear as fragmented filaments that display helicity when stained with antibody-conjugated fluorescent dyes (Mauriello et al., 2010). MreB filaments from M. xanthus are likely to differ from homologues from some other bacteria as helical MreB was not observed in Bacillus subtilis and E. coli (Dominguez-Escobar et al., 2011, Garner et al., 2011, van Teeffelen et al., 2011). Insights on MreB, such as its structure, dynamics and interaction with the gliding complex will provide critical information for understanding the mechanism of gliding. Importantly, since MreB is also a central player in cell wall synthesis (Errington, 2015), M. xanthus MreB must possess unique versatility to operate on different spatial and temporal scales to orchestrate multiple functions within the same cell.
Flavobacterial gliding couples a rotary motor to a unique secretion system
Many members of the phylum Bacteroidetes, including the model organism Flavobacterium johnsoniae (previously known as Cytophaga johnsonae), move by gliding motility. The shape and size of F. johnsoniae cells are similar to that of M. xanthus. However, F. johnsoniae glides about 50 times faster than M. xanthus and can move on a much wider range of surfaces (McBride & Nakane, 2015). F. johnsoniae cells, besides gliding along their long axes, sometimes lift one end off a glass surface, rotate their cell bodies around the other end (pivoting) or flip their cell bodies over (Lapidus & Berg, 1982). PMF was determined to be the energy source for flavobacterial gliding (Pate & Chang, 1979). However, the gliding motors in F. johnsoniae have still not been identified, in part because the function of the putative gliding motors seems to overlap with a unique protein secretion channel, designated as the type IX secretion system (T9SS) (McBride & Nakane, 2015).
Proteins identified as required for gliding are predicted to form several structural units: a) SprB and RemA, the surface adhesins that move rapidly on cell surfaces, driven by the putative gliding motor (Nakane et al., 2013, Shrivastava et al., 2012), b) an ABC transporter and c) a T9SS that secretes proteins including SprB and RemA (Braun et al., 2005, Nelson et al., 2008, Rhodes et al., 2011, Shrivastava et al., 2012, Shrivastava et al., 2013, McBride & Nakane, 2015). The ABC transporter is not likely to be the motor because it is not conserved in all gliding Bacteroidetes (McBride & Zhu, 2013). In contrast, the proteins that have the ability to harvest PMF were predicted to reside in the T9SS (McBride & Nakane, 2015). If this is the case, the gliding motor of F. johnsoniae might be analogous to the bacterial flagella motor in which PMF drives both the rotation of flagella and the secretion of flagellar proteins through a type III secretion system (Minamino et al., 2008, Paul et al., 2008).
Although the gliding motors of F. johnsoniae have not yet been identified, its function can be monitored through the motion of SprB and RemA. Images obtained using cryo-electron tomography showed that SprB forms 150-nm-long filaments that protrude to the cell surface from a “baseplate” structure underneath the outer membrane (Liu et al., 2007). SprB filaments labeled with fluorescent antibodies or latex beads move along helical trajectories at constant velocity (Nakane et al., 2013). When Shrivastava et al. used SprB antibodies to tether F. johnsoniae cells onto glass slides through single SprB filaments, the tethered cells spun around a fixed point at a constant angular speed of 1 Hz. These cellular movements presumably reflect the rotation of individual motor units. This observation indicates that F. johnsoniae gliding motors rotate in place (Shrivastava et al., 2015). Surprisingly, when the tethered cells were exposed to viscous media, their spinning speed remained unchanged. Thus, F. johnsoniae gliding motors appear to generate different torques (200-6,000 pN nm) at constant speed (Shrivastava et al., 2015), which differs from the flagella motor of E. coli that reduces speed to generate higher torque (Chen & Berg, 2000).
How is the in situ rotation of the motors transformed into the translational motion of SprB and the forward movement of cells? Several hypotheses have been suggested. The baseplate relay model speculates that one motor unit propels a baseplate on which adhesins such as SprB attach, until the baseplate is engaged by another motor. Thus, if many motors and baseplates line up along a helical track, the rotation of motors will pass adhesins along the track (Nan et al., 2014) (Fig. 1B). Another model pictures the adhesion filaments being carried by a continuous conveyor belt, driven by two rotary motors. Several conveyor belts may be arranged in patterns that connect to each other like treads that link sprockets in a snowmobile; this might give the movement of adhesins a helical appearance. Unlike the baseplate relay model, this “snowmobile” model would only require a few motor units (Shrivastava & Berg, 2015) (Fig. 1B). Progress in solving the puzzle of F. johnsoniae gliding should be forthcoming with the identification of the gliding motors, their number, and the respective functions of the motors and the T9SS.
Mycoplasma mobile gliding utilizes tiny legs marching unitarily
Mollicutes, including Mycoplasma, Spiroplasma and Achoreplasma, are parasitic or commensal bacteria that have very small genomes (Razin et al., 1998). They are related to the Gram-positive Firmicutes but lack peptidoglycan. Many Mollicutes species glide on sialyated ologisacharides (SO), major components on the surfaces of animal tissues (Kasai et al., 2013), but the mechanisms of gliding are not necessarily conserved across the class (Miyata & Hamaguchi, 2015).
The gliding mechanism of M. mobile, a fish pathogen, has been studied in great detail. M. mobile forms a membrane protrusion at one cell pole, giving it a unique cell shape similar to a bowling pin. The gliding machinery of M. mobile generates a force up to 27 pN, which enables cells to glide smoothly on a broad range of surfaces at a speed of 2.0-4.5 μm/s (Miyata et al., 2002). The M. mobile cell surface is covered by membrane-anchored proteins (Wu & Miyata, 2012). Examination of cells by electron microscopy revealed spike-like structures approximately 50 nm in length around the neck area of the bowling-pin-shaped cells (Miyata & Petersen, 2004). These structures are required for gliding motility and appear to function as tiny legs. Three proteins that localize near the neck, Gli123, Gli349 and Gli521 were identified as essential components in the motility machinery (Seto et al., 2005, Uenoyama et al., 2004, Uenoyama & Miyata, 2005b). Among these proteins, Gli349 forms the leg seen under EM, which binds SO directly (Adan-Kubo et al., 2006, Lesoil et al., 2010); Gli521 was proposed to function as a “crank” that connects Gli349 with Gli123 (Uenoyama et al., 2009, Seto et al., 2005, Nonaka et al., 2010); while Gli123 might function as a “mount” that determines the cellular localization of Gli349 and Gli521 (Uenoyama & Miyata, 2005b) (Fig. 1C). Each M. mobile cell contains around 450 legs in the neck region, which probably localize in a two-dimension matrix (Uenoyama & Miyata, 2005b). In fact, when the membrane was completely stripped by detergent, a cytoskeletal “jellyfish” structure became visible under EM, which contains an oval solid “bell” localized to the small tip of the bowling-pin-shaped cell and dozens of “tentacles” that extend from the bell to the neck region. Genetic studies suggested that this cytoskeletal structure is connected to the Gli123-Gli349-Gli521 gliding unit (Nakane & Miyata, 2007) (Fig. 1C).
M. mobile hydrolyzes ATP as the energy source for gliding. When M. mobile cells are treated with detergent and nucleases, they lose most cellular contents. However, these “ghost” cells are able to resume gliding when ATP is added (Uenoyama & Miyata, 2005a). The ATPase that drives M. mobile gliding has not yet been identified. Two proteins in the jellyfish cytoskeletal structure are homologous to the α- and β-subunits of the F1-ATPase, which may function as the motor (Nakane & Miyata, 2007).
Due to the extremely small size of M. mobile cells (<1 μm in length), it is technically very difficult to directly observe the gliding units in action. However, indirect observations have provided many clues for the gliding mechanism. For example, in artificially elongated cells, axial variations during gliding were magnified and a repeated pivoting of cell bodies was observed, suggesting that different gliding units function independently (Nakane & Miyata, 2012). Adding excess SO into cell suspensions reduced the number of legs that bind to the surface. Combining this method with high precision co-localization microscopy showed that cells move in unitary 70-nm steps, which might correspond to the strokes of single gliding units (Kinosita et al., 2014).
A centipede model (also called power stroke model) was proposed to explain the gliding mechanism of M. mobile. In this model, each Gli123-Gli349-Gli521 gliding unit undergoes a four-stroke mechanical cycle: the Gli349 leg catches SO molecules on the surface, the leg pulls back using ATP hydrolysis as energy, the cell body is dragged forward, then the leg is released from the surface (Miyata & Hamaguchi, 2015) (Fig. 1C).
Currently, a bottleneck in the research of gliding mycoplasmas is the lack of a robust method for site-directed genetic manipulations. Once such a method is available, the ATPase that actually powers the gliding units may be identified. Biochemical and biophysical approaches may also reveal additional details of mycoplasma gliding. For example, it may be possible to reconstitute the gliding machinery in vitro by assembling purified proteins or by stripping the cellular components that are not required for gliding from the ghost cells. Super-resolution microscopy and optical trapping might also be useful to study the motion of single gliding units.
Conclusion
The gliding mechanisms reviewed here are only a few examples of the diverse ways that bacteria move on surfaces (Jarrell & McBride, 2008). These mechanisms have blurred our definition of motility machineries. On the one hand, novel mechanisms might have evolved through the reconfiguration and repurposing of unrelated structures, such as myxobacterial proton channels and cytoskeletal elements, flavobacterial secretion channels and adhesins, and M. mobile's ATPase and adhesins. On the other hand, common motility structures such as flagella and pili could also be modified for novel functions. For example, new evidence suggests that some filamentous cyanobacteria modify Type IV pili to push instead of pull cells forward (Khayatan et al., 2015). Future studies on surface motility and the associated motors may inform strategies to control bacterial infections and may yield insights into the design of novel molecular machines.
Acknowledgements
We thank Daisuke Nakane and Abhishek Shrivastava for helpful discussion and critical reading of this manuscript. Our research is supported by the National Institute of Health Grant GM020509 to D.R.Z. and a Texas A&M University Startup funding to B.N.
References
- Adan-Kubo J, Uenoyama A, Arata T, Miyata M. Morphology of isolated Gli349, a leg protein responsible for Mycoplasma mobile gliding via glass binding, revealed by rotary shadowing electron microscopy. J Bacteriol. 2006;188:2821–2828. doi: 10.1128/JB.188.8.2821-2828.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagam R, Litwin DB, Czerwinski F, Sun M, Kaplan HB, Shaevitz JW, Igoshin OA. Myxococcus xanthus gliding motors are elastically coupled to the substrate as predicted by the focal adhesion model of gliding motility. PLoS Comput Biol. 2014;10:e1003619. doi: 10.1371/journal.pcbi.1003619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun TF, Khubbar MK, Saffarini DA, McBride MJ. Flavobacterium johnsoniae gliding motility genes identified by mariner mutagenesis. J Bacteriol. 2005;187:6943–6952. doi: 10.1128/JB.187.20.6943-6952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YW, Rettberg LA, Treuner-Lange A, Iwasa J, Sogaard-Andersen L, Jensen GJ. Architecture of the type IVa pilus machine. Science. 2016;351:aad2001. doi: 10.1126/science.aad2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Berg HC. Torque-speed relationship of the flagellar rotary motor of Escherichia coli. Biophys J. 2000;78:1036–1041. doi: 10.1016/S0006-3495(00)76662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Escobar J, Chastanet A, Crevenna AH, Fromion V, Wedlich-Soldner R, Carballido-Lopez R. Processive movement of MreB-associated cell wall biosynthetic complexes in bacteria. Science. 2011;333:225–228. doi: 10.1126/science.1203466. [DOI] [PubMed] [Google Scholar]
- Dubreuil JD, Giudice GD, Rappuoli R. Helicobacter pylori interactions with host serum and extracellular matrix proteins: potential role in the infectious process. Microbiol Mol Biol Rev. 2002;66:617–629. doi: 10.1128/MMBR.66.4.617-629.2002. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J. Bacterial morphogenesis and the enigmatic MreB helix. Nat Rev Microbiol. 2015;13:241–248. doi: 10.1038/nrmicro3398. [DOI] [PubMed] [Google Scholar]
- Garner EC, Bernard R, Wang W, Zhuang X, Rudner DZ, Mitchison T. Coupled, circumferential motions of the cell wall synthesis machinery and MreB filaments in B. subtilis. Science. 2011;333:222–225. doi: 10.1126/science.1203285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrichsen J. Bacterial surface translocation: a survey and a classification. Bacteriol Rev. 1972;36:478–503. doi: 10.1128/br.36.4.478-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J, Kaiser D. Genetics of gliding motility in Myxococcus xanthus (Myxobacterales): Two gene systems control movement. Mol. Gen. Genet. 1979;171:177–191. [Google Scholar]
- Jakobczak B, Keilberg D, Wuichet K, Sogaard-Andersen L. Contact- and Protein Transfer-Dependent Stimulation of Assembly of the Gliding Motility Machinery in Myxococcus xanthus. PLoS Genet. 2015;11:e1005341. doi: 10.1371/journal.pgen.1005341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell KF, McBride MJ. The surprisingly diverse ways that prokaryotes move. Nat Rev Microbiol. 2008;6:466–476. doi: 10.1038/nrmicro1900. [DOI] [PubMed] [Google Scholar]
- Kasai T, Nakane D, Ishida H, Ando H, Kiso M, Miyata M. Role of binding in Mycoplasma mobile and Mycoplasma pneumoniae gliding analyzed through inhibition by synthesized sialylated compounds. J Bacteriol. 2013;195:429–435. doi: 10.1128/JB.01141-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane R, Berleman J. The predatory life cycle of Myxococcus xanthus. Microbiology. 2016;162:1–11. doi: 10.1099/mic.0.000208. [DOI] [PubMed] [Google Scholar]
- Kearns DB. A field guide to bacterial swarming motility. Nat Rev Microbiol. 2010;8:634–644. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayatan B, Meeks JC, Risser DD. Evidence that a modified type IV pilus-like system powers gliding motility and polysaccharide secretion in filamentous cyanobacteria. Mol Microbiol. 2015;98:1021–1036. doi: 10.1111/mmi.13205. [DOI] [PubMed] [Google Scholar]
- Kinosita Y, Nakane D, Sugawa M, Masaike T, Mizutani K, Miyata M, Nishizaka T. Unitary step of gliding machinery in Mycoplasma mobile. Proc Natl Acad Sci U S A. 2014;111:8601–8606. doi: 10.1073/pnas.1310355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus IR, Berg HC. Gliding motility of Cytophaga sp. strain U67. J Bacteriol. 1982;151:384–398. doi: 10.1128/jb.151.1.384-398.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesoil C, Nonaka T, Sekiguchi H, Osada T, Miyata M, Afrin R, Ikai A. Molecular shape and binding force of Mycoplasma mobile's leg protein Gli349 revealed by an AFM study. Biochem Biophys Res Commun. 2010;391:1312–1317. doi: 10.1016/j.bbrc.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Li Y, Sun H, Ma X, Lu A, Lux R, Zusman D, Shi W. Extracellular polysaccharides mediate pilus retraction during social motility of Myxococcus xanthus. Proc Natl Acad Sci U S A. 2003;100:5443–5448. doi: 10.1073/pnas.0836639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, McBride MJ, Subramaniam S. Cell surface filaments of the gliding bacterium Flavobacterium johnsoniae revealed by cryo-electron tomography. J Bacteriol. 2007;189:7503–7506. doi: 10.1128/JB.00957-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano J, Agrebi R, Le Gall AV, Wartel M, Fiegna F, Ducret A, Brochier-Armanet C, Mignot T. Emergence and modular evolution of a novel motility machinery in bacteria. PLoS Genet. 2011;7:e1002268. doi: 10.1371/journal.pgen.1002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunsdorf H, Schairer HU. Frozen motion of gliding bacteria outlines inherent features of the motility apparatus. Microbiology. 2001;147:939–947. doi: 10.1099/00221287-147-4-939. [DOI] [PubMed] [Google Scholar]
- Maier B, Potter L, So M, Long CD, Seifert HS, Sheetz MP. Single pilus motor forces exceed 100 pN. Proc Natl Acad Sci U S A. 2002;99:16012–16017. doi: 10.1073/pnas.242523299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley S, Gillette JM, Patterson GH, Shroff H, Hess HF, Betzig E, Lippincott-Schwartz J. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat Methods. 2008;5:155–157. doi: 10.1038/nmeth.1176. [DOI] [PubMed] [Google Scholar]
- Mauriello EM, Mouhamar F, Nan B, Ducret A, Dai D, Zusman DR, Mignot T. Bacterial motility complexes require the actin-like protein, MreB and the Ras homologue, MglA. Embo J. 2010;29:315–326. doi: 10.1038/emboj.2009.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride MJ, Nakane D. Flavobacterium gliding motility and the type IX secretion system. Curr Opin Microbiol. 2015;28:72–77. doi: 10.1016/j.mib.2015.07.016. [DOI] [PubMed] [Google Scholar]
- McBride MJ, Zhu Y. Gliding motility and Por secretion system genes are widespread among members of the phylum bacteroidetes. J Bacteriol. 2013;195:270–278. doi: 10.1128/JB.01962-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot T, Shaevitz JW, Hartzell PL, Zusman DR. Evidence that focal adhesion complexes power bacterial gliding motility. Science. 2007;315:853–856. doi: 10.1126/science.1137223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, Imada K, Namba K. Molecular motors of the bacterial flagella. Curr Opin Struct Biol. 2008;18:693–701. doi: 10.1016/j.sbi.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Miyata M, Hamaguchi T. Prospects for the gliding mechanism of Mycoplasma mobile. Curr Opin Microbiol. 2015;29:15–21. doi: 10.1016/j.mib.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Miyata M, Petersen JD. Spike structure at the interface between gliding Mycoplasma mobile cells and glass surfaces visualized by rapid-freeze-and-fracture electron microscopy. J Bacteriol. 2004;186:4382–4386. doi: 10.1128/JB.186.13.4382-4386.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata M, Ryu WS, Berg HC. Force and velocity of Mycoplasma mobile gliding. J Bacteriol. 2002;184:1827–1831. doi: 10.1128/JB.184.7.1827-1831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane D, Miyata M. Cytoskeletal “jellyfish” structure of Mycoplasma mobile. Proc Natl Acad Sci U S A. 2007;104:19518–19523. doi: 10.1073/pnas.0704280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane D, Miyata M. Mycoplasma mobile cells elongated by detergent and their pivoting movements in gliding. J Bacteriol. 2012;194:122–130. doi: 10.1128/JB.05857-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane D, Sato K, Wada H, McBride MJ, Nakayama K. Helical flow of surface protein required for bacterial gliding motility. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1219753110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan B, Bandaria JN, Guo KY, Fan X, Moghtaderi A, Yildiz A, Zusman DR. The polarity of myxobacterial gliding is regulated by direct interactions between the gliding motors and the Ras homolog MglA. Proc Natl Acad Sci U S A. 2015;112:E186–193. doi: 10.1073/pnas.1421073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan B, Bandaria JN, Moghtaderi A, Sun IH, Yildiz A, Zusman DR. Flagella stator homologs function as motors for myxobacterial gliding motility by moving in helical trajectories. Proc Natl Acad Sci U S A. 2013;110:E1508–1513. doi: 10.1073/pnas.1219982110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan B, Chen J, Neu JC, Berry RM, Oster G, Zusman DR. Myxobacteria gliding motility requires cytoskeleton rotation powered by proton motive force. Proc Natl Acad Sci U S A. 2011;108:2498–2503. doi: 10.1073/pnas.1018556108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan B, Mauriello EM, Sun IH, Wong A, Zusman DR. A multi-protein complex from Myxococcus xanthus required for bacterial gliding motility. Mol Microbiol. 2010;76:1539–1554. doi: 10.1111/j.1365-2958.2010.07184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan B, McBride MJ, Chen J, Zusman DR, Oster G. Bacteria that glide with helical tracks. Curr Biol. 2014;24:R169–R173. doi: 10.1016/j.cub.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan B, Zusman DR. Uncovering the mystery of gliding motility in the myxobacteria. Annu Rev Genet. 2011;45:21–39. doi: 10.1146/annurev-genet-110410-132547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SS, Bollampalli S, McBride MJ. SprB is a cell surface component of the Flavobacterium johnsoniae gliding motility machinery. J Bacteriol. 2008;190:2851–2857. doi: 10.1128/JB.01904-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka T, Adan-Kubo J, Miyata M. Triskelion structure of the Gli521 protein, involved in the gliding mechanism of Mycoplasma mobile. J Bacteriol. 2010;192:636–642. doi: 10.1128/JB.01143-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate JL, Chang L-YE. Evidence that gliding motility in prokaryotic cells is driven by rotary assemblies in the cell envelopes. Curr. Microbiol. 1979;2:59–64. [Google Scholar]
- Paul K, Erhardt M, Hirano T, Blair DF, Hughes KT. Energy source of flagellar type III secretion. Nature. 2008;451:489–492. doi: 10.1038/nature06497. [DOI] [PubMed] [Google Scholar]
- Pelling AE, Li Y, Shi W, Gimzewski JK. Nanoscale visualization and characterization of Myxococcus xanthus cells with atomic force microscopy. Proc Natl Acad Sci U S A. 2005;102:6484–6489. doi: 10.1073/pnas.0501207102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persat A, Nadell CD, Kim MK, Ingremeau F, Siryaporn A, Drescher K, Wingreen NS, Bassler BL, Gitai Z, Stone HA. The mechanical world of bacteria. Cell. 2015;161:988–997. doi: 10.1016/j.cell.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes RG, Nelson SS, Pochiraju S, McBride MJ. Flavobacterium johnsoniae sprB is part of an operon spanning the additional gliding motility genes sprC, sprD, and sprF. J Bacteriol. 2011;193:599–610. doi: 10.1128/JB.01203-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto S, Uenoyama A, Miyata M. Identification of a 521-kilodalton protein (Gli521) involved in force generation or force transmission for Mycoplasma mobile gliding. J Bacteriol. 2005;187:3502–3510. doi: 10.1128/JB.187.10.3502-3510.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava A, Berg HC. Towards a model for Flavobacterium gliding. Curr Opin Microbiol. 2015;28:93–97. doi: 10.1016/j.mib.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava A, Johnston JJ, van Baaren JM, McBride MJ. Flavobacterium johnsoniae GldK, GldL, GldM, and SprA are required for secretion of the cell surface gliding motility adhesins SprB and RemA. J Bacteriol. 2013;195:3201–3212. doi: 10.1128/JB.00333-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava A, Lele PP, Berg HC. A rotary motor drives Flavobacterium gliding. Curr Biol. 2015;25:338–341. doi: 10.1016/j.cub.2014.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava A, Rhodes RG, Pochiraju S, Nakane D, McBride MJ. Flavobacterium johnsoniae RemA is a mobile cell surface lectin involved in gliding. J Bacteriol. 2012;194:3678–3688. doi: 10.1128/JB.00588-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerker JM, Berg HC. Direct observation of extension and retraction of type IV pili. Proc Natl Acad Sci U S A. 2001;98:6901–6904. doi: 10.1073/pnas.121171698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilenov LB, Mikhailov A, Pelham RJ, Marcantonio EE, Gundersen GG. Focal adhesion motility revealed in stationary fibroblasts. Science. 1999;286:1172–1174. doi: 10.1126/science.286.5442.1172. [DOI] [PubMed] [Google Scholar]
- Sun M, Wartel M, Cascales E, Shaevitz JW, Mignot T. Motor-driven intracellular transport powers bacterial gliding motility. Proc Natl Acad Sci U S A. 2011;108:7559–7564. doi: 10.1073/pnas.1101101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treuner-Lange A, Macia E, Guzzo M, Hot E, Faure LM, Jakobczak B, Espinosa L, Alcor D, Ducret A, Keilberg D, Castaing JP, Lacas Gervais S, Franco M, Sogaard-Andersen L, Mignot T. The small G-protein MglA connects to the MreB actin cytoskeleton at bacterial focal adhesions. J Cell Biol. 2015;210:243–256. doi: 10.1083/jcb.201412047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uenoyama A, Kusumoto A, Miyata M. Identification of a 349-kilodalton protein (Gli349) responsible for cytadherence and glass binding during gliding of Mycoplasma mobile. J Bacteriol. 2004;186:1537–1545. doi: 10.1128/JB.186.5.1537-1545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uenoyama A, Miyata M. Gliding ghosts of Mycoplasma mobile. Proc Natl Acad Sci U S A. 2005a;102:12754–12758. doi: 10.1073/pnas.0506114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uenoyama A, Miyata M. Identification of a 123-kilodalton protein (Gli123) involved in machinery for gliding motility of Mycoplasma mobile. J Bacteriol. 2005b;187:5578–5584. doi: 10.1128/JB.187.16.5578-5584.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uenoyama A, Seto S, Nakane D, Miyata M. Regions on Gli349 and Gli521 protein molecules directly involved in movements of Mycoplasma mobile gliding machinery, suggested by use of inhibitory antibodies and mutants. J Bacteriol. 2009;191:1982–1985. doi: 10.1128/JB.01012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Teeffelen S, Wang S, Furchtgott L, Huang KC, Wingreen NS, Shaevitz JW, Gitai Z. The bacterial actin MreB rotates, and rotation depends on cell-wall assembly. Proc Natl Acad Sci U S A. 2011;108:15822–15827. doi: 10.1073/pnas.1108999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HN, Miyata M. Whole surface image of Mycoplasma mobile, suggested by protein identification and immunofluorescence microscopy. J Bacteriol. 2012;194:5848–5855. doi: 10.1128/JB.00976-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SS, Kaiser D. Genetic and functional evidence that Type IV pili are required for social gliding motility in Myxococcus xanthus. Mol Microbiol. 1995;18:547–558. doi: 10.1111/j.1365-2958.1995.mmi_18030547.x. [DOI] [PubMed] [Google Scholar]
- Youderian P, Burke N, White DJ, Hartzell PL. Identification of genes required for adventurous gliding motility in Myxococcus xanthus with the transposable element mariner. Mol Microbiol. 2003;49:555–570. doi: 10.1046/j.1365-2958.2003.03582.x. [DOI] [PubMed] [Google Scholar]
- Zusman DR, Scott AE, Yang Z, Kirby JR. Chemosensory pathways, motility and development in Myxococcus xanthus. Nat Rev Microbiol. 2007;5:862–872. doi: 10.1038/nrmicro1770. [DOI] [PubMed] [Google Scholar]