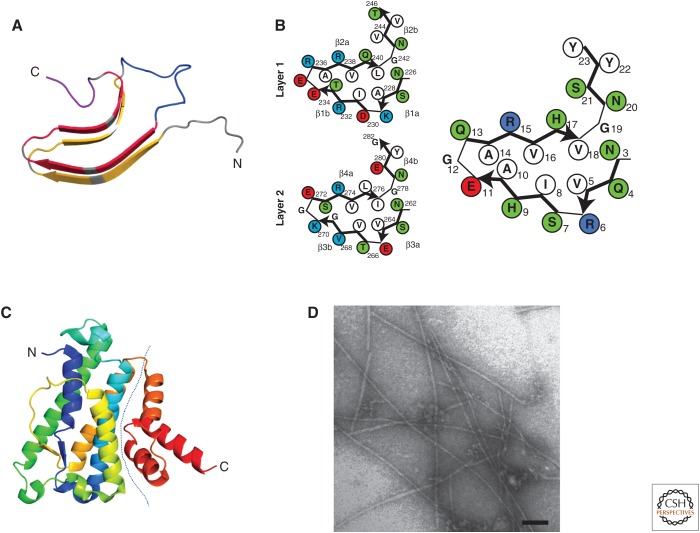
Figure 2.
HET-S/s prion-forming domain (PFD) and HeLo domain structure. (A) The β-solenoid structure of HET-s(218–289). The structure of one monomer in the fibrillar form is shown (after PDB 2KJ3). Each rung of β-strands is shown in yellow and the second is in red. The connecting central loop is shown in blue, and the C-terminal loop forming the semi-hydrophobic pocket is in magenta. (B) Individual β-solenoid motifs of HET-s and NWD2. A schematic representation of the β-solenoid form of repeats 1 and 2 of HET-s and NWD2(3–23) are given on the left and right, respectively. The NWD2(3–23) model is based on homology modeling and solid-state nuclear magnetic resonance (NMR) (see text for details). (C) The structure of the HeLo domain of HET-S (after PDB 2WVO). The dotted line delimits the N-terminal and C-terminal bundles forming subdomains. (D) Electron micrograph of NWD2(3–23) fibrils. Scale bar, 50 nm.