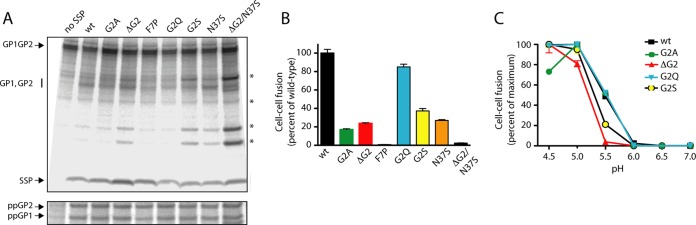
FIG 5.
Biochemical and functional characterization of myristoylation mutants in ectopically expressed GPC. (A) Vero cells expressing the wild-type and mutant GPCs were metabolically labeled, and lysates were subjected to immunoprecipitation using the GP1-directed MAb BF11 (38). In these studies, the mutant SSPs were expressed in trans with the wild-type GP1/GP2 precursor (fused to the conventional signal peptide of human CD4) to reconstitute native GPC (18, 37). Proteins were resolved by SDS-PAGE, and incorporated [35S]methionine-cysteine was imaged using a Fuji FLA 3000G imager. In addition to the indicated mutants, a sample in which SSP was omitted from the transfection (no SSP) is included to draw attention to SSP association and the presence of the mature GP1 and GP2 subunits in the mutant GPCs. The GP1/GP2 precursor is not proteolytically cleaved in the absence of SSP. The positions of the GP1/GP2 precursor, SSP, and the comigrating GP1 and GP2 subunits are marked. Additional bands of unknown origin are indicated by asterisks (*). In the bottom panel, the immunoprecipitated protein was deglycosylated using peptide N-glycosidase F (New England BioLabs) to resolve the GP1 and GP2 polypeptides (ppGP1 and ppGP2, respectively). (B) The relative cell-cell fusion activities of the GPC mutants were determined using a vaccinia virus-based β-galactosidase fusion reporter assay as described in Materials and Methods. Fusion was induced by exposing the cell monolayer to medium adjusted to pH 5.0, the optimal pH for wild-type GPC fusion. (C) The pH profile for cell-cell fusion was determined by varying the pH of the acidic medium, as indicated. All curves were normalized to the maximum extent of fusion by each GPC (100%).