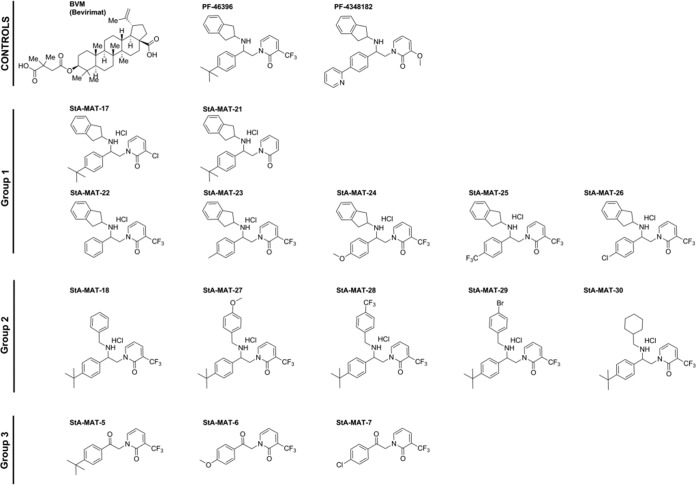
FIG 1.
Chemical structures of BVM, PF-46396, and analogues. Controls are the maturation inhibitors BVM, PF-46396, and PF-4348182, which is an inactive analogue with a replacement of the tert-butyl group on the phenyl ring with a 2-pyridyl group and a replacement of the trifluoromethyl group on the pyridone core with a methoxy group. The test analogue series consisted of three groups. Group 1 analogues had a replacement of the trifluoromethyl group on the pyridone core with a chloro group (StA-MAT-17) or no substituent (StA-MAT-21) or a replacement of the tert-butyl group on the phenyl ring with no substituent (StA-MAT-22), a methyl group (StA-MAT-23), a methoxy group (StA-MAT-24), a trifluoromethyl group (StA-MAT-25), or a chloro group (StA-MAT-26). Group 2 analogues had the 2-aminoindan group replaced with a benzylamine fragment with no substituent on the benzyl ring (StA-MAT-18) or with a replacement at position 4 of the benzyl ring with a methoxy group (StA-MAT-27), a trifluoromethoxy group (StA-MAT-28), or a bromo group (StA-MAT-29) or had the 2-aminoindan group replaced with an (aminomethyl)cyclohexane group (StA-MAT-30). Group 3 contains analogues with no 2-aminoindan group (StA-MAT-5) or no 2-aminoindan group and a replacement of the tert-butyl group on the phenyl ring with a methoxy group (StA-MAT-6) or a chloro group (StA-MAT-7).