Abstract
Movement atypicalities in speed, coordination, posture, and gait have been observed across the autism spectrum (AS) and atypicalities in coordination are more commonly observed in AS individuals without delayed speech (DSM‐IV Asperger) than in those with atypical or delayed speech onset. However, few studies have provided quantitative data to support these mostly clinical observations. Here, we compared perceptual and motor performance between 30 typically developing and AS individuals (21 with speech delay and 18 without speech delay) to examine the associations between limb movement control and atypical speech development. Groups were matched for age, intelligence, and sex. The experimental design included: an inspection time task, which measures visual processing speed; the Purdue Pegboard, which measures finger dexterity, bimanual performance, and hand‐eye coordination; the Annett Peg Moving Task, which measures unimanual goal‐directed arm movement; and a simple reaction time task. We used analysis of covariance to investigate group differences in task performance and linear regression models to explore potential associations between intelligence, language skills, simple reaction time, and visually guided movement performance. AS participants without speech delay performed slower than typical participants in the Purdue Pegboard subtests. AS participants without speech delay showed poorer bimanual coordination than those with speech delay. Visual processing speed was slightly faster in both AS groups than in the typical group. Altogether, these results suggest that AS individuals with and without speech delay differ in visually guided and visually triggered behavior and show that early language skills are associated with slower movement in simple and complex motor tasks. Autism Res 2015, 8: 682–693. © 2015 The Authors Autism Research published by Wiley Periodicals, Inc. on behalf of International Society for Autism Research
Keywords: autism spectrum, Asperger syndrome, motor skills, motor control, coordination, speech onset delay
Introduction
Movement atypicalities are commonly observed in individuals across the autism spectrum (AS). Although they are not included in the DSM‐IV [APA, 1994] diagnostic criteria, the Autism Diagnostic Interview [ADI; Lord, Rutter, & Le Couteur, 1994], includes questions about motor skills, such as gait, posture, and coordination. The DSM‐5 [APA, 2013] lists developmental motor or coordination disorders as independent conditions that can be associated.
AS has been associated with a range of movement atypicalities, including atypical coordination, posture, and voluntary movement speed [Dziuk et al., 2007; Mostofsky, Burgess, & Gidley Larson, 2007; Rinehart, Bradshaw, Brereton, & Tonge, 2001]. Mechanisms accounting for these difficulties may include problems with movement planning [Forti et al., 2011; Hughes, 1996], movement anticipation [Brisson, Warreyn, Serres, Foussier, & Adrien, 2012], movement preparation and initiation [Glazebrook, Elliott, & Lyons, 2006; Rinehart et al., 2006, 2001] and feed‐forward control mechanisms [Stoit, van Schie, H. T., Slaats‐Willemse, & Buitelaar, 2013]. Atypical perceptual processing is a frequently suggested cause for movement atypicalities associated with autism [Fournier, Hass, Naik, Lodha, & Cauraugh, 2010; Whyatt & Craig, 2012], because it can affect perceptual input and visual‐motor integration [Dowd, McGinley, Taffe, & Rinehart, 2011; Linkenauger, Lerner, Ramenzoni, & Proffitt, 2012; Mayes & Calhoun, 2007]. When describing altered motor behavior in autism, Gowen and Hamilton [2013] concluded that the “basic motor machinery”, including motor learning and motor adaptation, was intact in autism. Instead, they propose that motor problems in AS involve perceptual inputs and are related to poor higher order sensorimotor integration, resulting in the slow planning of movements.
Moreover, it is unclear whether movement atypicalities are uniformly distributed across the entire AS, and how the recently introduced clinical DSM‐5 specifiers of intelligence, associated conditions, language level, and severity, are related to motor performance. One of the most frequently reported factors influencing the presence and nature of movement atypicalities in AS is language, in particular the time of speech onset. For instance, DSM‐IV [APA, 1994] includes “motor delays or motor clumsiness” in the Asperger syndrome (i.e., AS with typical speech onset) diagnostic criteria, and only “abnormalities of posture” in autism (i.e., AS with delayed speech onset). The European mental health classification system, ICD‐10, also includes motor “clumsiness” as a diagnostic criterion for Asperger syndrome but not for autism [WHO, 1992].
Some empirical evidences suggest that DSM‐IV Asperger syndrome individuals display more movement atypicalities than autistic individuals [Macintosh & Dissanayake, 2004 for a review]. However, studies examining the differential occurrence of movement atypicalities in autistic individuals with or without speech delay have produced inconsistent results. Behere, Shahani, Noggle, & Dean [2012] argued that motor performance could be used to classify AS subgroups; although movement atypicalities are present in both DSM‐IV autism and Asperger syndrome, different neural mechanisms may be responsible for atypical movements observed in each condition. Gross motor skills [Gillberg, 1998], fine and gross motor abilities [Klin, Volkmar, Sparrow, Cicchetti, & Rourke, 1995], and the speed and dexterity of non‐dominant hand movements [Szatmari, Tuff, Finlayson, & Bartolucci, 1990] have been suggested to be more impaired in individuals with Asperger syndrome than in autism. If both autism and Asperger syndrome are associated with atypicalities in movement preparation, autistic individuals may specifically lack anticipation before initiating actions, whereas individuals with Asperger syndrome may exhibit more general deficits in movement preparation [Rinehart et al., 2001]. Furthermore, autistic individuals may have more problems in saccade adaptation than individuals with Asperger syndrome, which could affect visually guided hand movement [Johnson, Rinehart, White, Millist, & Fielding, 2013], voluntary saccade control [Stanley‐Cary, Rinehart, Tonge, White, & Fielding, 2011] and gait [Nayate et al., 2012]. However, other evidence suggests that individuals with autism or Asperger syndrome show a similar level of movement atypicalities, including abilities such as manual dexterity, static and dynamic balance, ball skills and repetitive timed movements [Jansiewicz et al., 2006; Manjiviona & Prior, 1995; Noterdaeme, Wriedt, & Hohne, 2010].
Movement atypicalities are associated with other DSM‐5 diagnostic specifiers besides language. For example, IQ is linked with variations in motor abilities [Green et al., 2009; Papadopoulos et al., 2012]. Individuals with intellectual disabilities often show movement atypicalities [Hartman, Houwen, Scherder, & Visscher, 2010; Westendorp, Houwen, Hartman, & Visscher, 2011], and intellectual functioning predicts visuomotor integration skills in autism (e.g., the ability to copy simple and complicated designs) [Memisevic & Sinanovic, 2012]. Even for autistic individuals in the normal intelligence range, movement atypicalities are related to intelligence [Smits‐Engelsman & Hill, 2012]. Fluid and quantitative reasoning, but not verbal abilities, are typically associated with visuomotor integration abilities [Decker, Englund, Carboni, & Brooks, 2011]. This observation is relevant when studying AS subgroups, because verbal and non‐verbal intelligence relate differently to other cognitive competencies such as processing speed in both DSM‐IV autism and Asperger syndrome [Barbeau, Soulieres, Dawson, Zeffiro, & Mottron, 2013]. This difference possibly results from the discrepancy observed in autistic individuals between performance on the Raven's Progressive Matrices (RPM) and Wechsler's IQ measures [Dawson, Soulieres, Gernsbacher, & Mottron, 2007], which is less pronounced in individuals with Asperger syndrome [Soulieres, Dawson, Gernsbacher, & Mottron, 2011], and typically developing individuals. The authors of these studies suggest that the RPM IQ estimate is a more appropriate measure than the Wechsler IQ to control for intelligence when studying differences in non‐verbal abilities between and within AS subgroups. We sought to understand better the interactions between speech development, limb motor control and intelligence in autism, in particular because atypical motor control can be an early sign of AS [Landa & Garrett‐Mayer, 2006; Teitelbaum et al., 2004]; therefore, we investigated perceptual and motor behavior in AS individuals differing in time of speech acquisition during development.
In this study, we used quantitative psychophysical and kinematic techniques (1) to explore further the association between motor control mechanisms and speech onset in AS, and (2) to examine the relationships between motor performance, visual processing speed and intelligence measures. AS participants were characterized by presence (SOD) or absence of developmental speech onset delay (NoSOD) and their motor performance was assessed. Our experimental approach involved behavioral analysis of tasks assessing visual perception, visually guided movements and visually triggered movements to identify the locus of processing atypicalities. The visually guided tasks used were the Annett Peg Moving Task [Annett, 2002], a unimanual task assessing goal‐directed movement, and the Purdue Pegboard, a task assessing fine motor abilities, dexterity, and bimanual coordination. We also used a visual inspection time task, which measures visual processing speed. Last, visually triggered movements were examined with a simple reaction time (SRT) task measuring the time required to press a button following the presentation of a brief visual stimulus.
Methods
Participants
All participants were randomly recruited from the research database of the Specialized Autism Clinic at the Rivière‐des‐Prairies Hospital (Montreal, Canada). The experimental group included 39 AS participants and 30 typically developing individuals (Table 1). Exclusion criteria included: uncorrectable visual impairment, use of drugs or alcohol exceeding two drinks per day, and FSIQ less than 75 (to ensure that all participants had normal intelligence). Two AS participants took antidepressants (paroxetine and venlaflaxine), one a sleep medication (fluorazepam), and three a stimulant (methylphenidate). Psychiatric comorbidities were clinically assessed by an experienced psychiatrist in standardized and nonstandardized conditions, in addition to an average of two professionals (speech therapist and neuropsychologist). None of the AS participants had any known comorbid genetic, neurological, or DSM‐IV Axis I psychiatric conditions, other than ADHD (two participants) and language disorders, which occur in a large proportion of AS individuals at some point during development. Additionally, typically developing comparison participants completed a questionnaire to screen for personal or familial neurological, psychiatric or medical conditions known to affect brain function. All participants gave written informed consent and were compensated for their participation.
Table 1.
Participant Characteristics
| AS‐NoSOD | AS‐SOD | Typical | P | |
|---|---|---|---|---|
| n | 18 (17M:1F) | 21 (19M:2F) | 30 (26M:4F) | |
| Age (SD) | 20.7 (4.9) | 22.8 (6.2) | 20.3 (4.6) | 0.228 |
| Range | 14–30 | 15–34 | 14–35 | |
| FSIQ (SD) | 103.8 (12.5) | 98.3 (13.8) | 105.0 (10.8) | 0.148 |
| Range | 86–129 | 78–120 | 80–121 | |
| PIQ (SD) | 98.0 (12.3) | 102.3 (8.2) | 101.8 (13.0) | 0.455 |
| Range | 75–126 | 91–118 | 72–122 | |
| VIQ (SD) | 108.7 (12.1) | 97.1 (17.5) | 107.9 (10.6) | 0.010 |
| Range | 94–134 | 72–124 | 91–127 | |
| RPM %tile (SD) | 78.9 (22.6) | 66.6 (28.8) | 63.8 (21.0) | 0.105 |
| Range | 25–98 | 9–98 | 23–96.5 | |
| Edinburgh (SD) | 56.5 (70.1) | 66.8 (62.7) | 32.1 (71.2) | 0.185 |
| Range | −100–100 | −100–100 | −100–100 |
Diagnostic Procedures
Twenty‐eight of the 39 AS participants were diagnosed by both the Autism Diagnostic Interview‐Revised [ADI‐R; Lord et al., 1994] and the Autism Diagnosis Observation Schedule module 3 or 4 [ADOS‐G; Lord et al., 2000], combined with an expert interdisciplinary clinical assessment. However, some participants were diagnosed by expert interdisciplinary judgment alone (one participant), or expert judgment combined with either the ADOS‐G (four participants) or the ADI‐R (six participants). Age of first words and first phrases were available for all AS participants.
AS Subgroups
The 39 AS participants were divided into two groups according to speech onset delay using the ADI‐R questions: age of first words (#9) and age of first phrases (#10). We used the term autism for participants with both a clinical diagnosis of autism and speech onset delay involving first words (≥24 months) or first phrases (≥33 months), as defined by the ADI. To be consistent with DSM‐5 terminology, we used the term AS‐SOD for AS individuals with speech onset delay, and AS‐NoSOD for individuals with a DSM‐IV clinical diagnosis of Asperger syndrome without speech onset delay. Participants with a clinical diagnosis that was not consistent with the corresponding absence or presence of speech onset delay (e.g., clinical diagnosis of autism but no speech delay) were excluded. The final sample consisted of 18 individuals with AS‐NoSOD and 21 AS‐SOD participants. The AS‐SOD, AS‐NoSOD, and typical groups were comparable in terms of age, full scale IQ, performance IQ, and RPM score; however, the AS‐SOD group had a lower VIQ than the other two groups, which was expected given that developmental speech delay was used in assigning groups.
Procedure
Handedness assessment
Manual preference was estimated by self‐report with the Edinburgh Handedness Inventory [Oldfield, 1971] and by observations in the Hand Preference Demonstration Test [Soper et al., 1986]. This test includes ten items assessing the subject's preferred hand during the performance of a wide range of activities involving miming ten actions (e.g., throwing a ball) and performing these actions with real objects (e.g., a ball). Items are presented twice within a session in a pseudorandom order. The two manual preference measures were consistent. The scores were strongly correlated in the whole sample (r = 0.950, P < 0.001) and within each group.
Motor skill assessment
The Annett Peg Moving Task [Annett, 2002] is a visually guided movement task that assesses unimanual goal‐directed movement by measuring the speed at which a set of ten pegs can be moved, one after another, from one row of holes to another. The wooden board includes two parallel rows of 10 holes (1.27‐cm diameter, 2.22‐cm deep, and 3.81‐cm apart) 20.3 cm apart. The pegs are 5.1 cm tall. Six timed trials were completed with each hand in a standing position. A trial was considered valid when no pegs were dropped and no significant distraction interfered. For each participant, the slowest trial of six for each hand was discarded [Annett, 2002]. The remaining five valid trials were then averaged for each hand. The three final measures of the Annett test were: average time (in sec) taken with the dominant hand (DH), the non‐dominant hand (NDH), and the average of all 10 trials (Total).
The Purdue Pegboard Test (Model 32020, Lafayette Instrument Co., IL) is a visually guided movement task that assesses fine goal‐directed movements by measuring fingertip dexterity, hand‐eye coordination, and bimanual coordination. The pieces and holes are much smaller than those of the Annett Peg Moving Task (2.5‐mm diameter, 25‐mm long) and have to be manipulated between two fingers, requiring greater precision and dexterity. The test measures the ability: (1) to put as many pegs as possible into the pegboard in 30 sec using the dominant hand (DH), the non‐dominant hand (NDH), or both hands simultaneously in a coordinated mirrored fashion (BH), and (2) to assemble washers, collars, and pegs in a specific sequence using both hands in a coordinated and sequential fashion within 60 sec (Assembly). Trials were completed three times each and averaged. The score for each of the four conditions (DH, NDH, BH, and Assembly) is recorded as the number of pieces placed within 30 sec. For both tasks, manual preference was controlled by classifying responses according to whether the dominant (DH) or non‐dominant hand (NDH) was used.
Simple reaction time
A visually triggered SRT measure was obtained to estimate movement speed in the context of simple movements. Participants were seated in a quiet, dimly lit room. Black wooden panels surrounded the participants, with an opening for the computer screen. A chin rest placed at 73 cm from the screen minimized head movements. A gray background with a centered white fixation cross was present throughout the task and the participants were instructed to maintain fixation. The visual stimulus was a small black square that appeared for 50 ms randomly either to the right or left of the fixation cross, at an eccentricity of 8 degrees of visual angle. The inter‐stimulus interval varied randomly from 1000 to 3500 ms to avoid anticipation effects. Participants were instructed to press a button as quickly as possible after detecting the black square. The session consisted of three right and three left hand blocks (about 4 min each) of 100 trials each, for a total of 600 trials. The order of blocks varied between participants.
Inspection time
We used the Inspection Time Task to investigate whether perceptual processing speed contributes to visually guided movement atypicalities observed in autism [Gepner & Feron, 2009]. In this task, two vertical lines of different lengths were presented for 10 to 200 ms and then were immediately masked by two irregular vertical shapes. Participants indicated which line was longer. Each stimulus was preceded by a fixation cross prompting the participant to look at the middle of the screen. The duration of each stimulus was individually and adaptively varied in a staircase psychophysical procedure. This procedure determines the minimal exposure time necessary to detect the difference in length between the two lines [see Barbeau et al., 2013 for a detailed method]. A valid measure of inspection time was available for 18 AS‐SOD, 21 AS‐NoSOD, and 30 typical participants; three AS‐SOD participants did not complete or failed to perform the task correctly.
Statistical Analysis
For the Annett Peg Moving Task and the Purdue Pegboard, we removed outlier scores that were two or more standard deviations (SD) above or below the group average. This made the response distributions more Gaussian and, therefore, more appropriate for the parametric statistical modeling that we used. Outliers were evenly distributed across groups (data points removed for Annett DH condition from 1 AS‐NoSOD, 1 typical participant; NDH: 1 AS‐NoSOD, 2 AS‐SODs; Purdue DH: 1 AS‐NoSOD; NDH: 1 AS‐NoSOD, 1 AS‐SOD; BH: 1 AS‐NoSOD; AS: 1 AS‐SOD, 2 typicals). Although one might want to exclude participants with known ADHD because of its link with motor difficulties, the two participants with ADHD (1 AS‐NoSOD and 1 AS‐SOD) were not excluded from most of the analyses because their performance fell within their respective subgroups' range. The statistical analyses were also performed using 3 SD as a cutoff to account for the possibility that the outliers removed were actually representative of the sample studied. This 3 SD cutoff excluded one AS‐NoSOD participant for the Annett only. For the SRT task, the response measure was obtained by calculating the median of all trials over 150 ms (shorter RTs were treated as anticipation errors or missed trials) and below 800 ms (longer RTs were treated as inattention errors).
Motor tasks
ANCOVAs were conducted for each condition of the Annett Peg Moving test (DH and NDH), the Purdue Pegboard (DH, NDH, BH, and Assembly), and the SRT task with SPSS 17.0.1 software (SPSS Inc., Chicago, IL). The RPM percentile score was used as a covariate in the analysis because of the known relationship between general intelligence and motor skills [Smits‐Engelsman & Hill, 2012]. Our AS groups were formed according to the presence or absence of developmental speech onset delay; therefore, we additionally examined the effects of Verbal IQ, by treating it as a covariate. The age of participants did not have a significant effect on any of the measures and it was not included in the model. Linear regression analyses were also conducted to investigate the relationship between motor skill measures and the age of first phrases in months and the SRT.
Inspection time
We used a mixed effects model with group as a between‐subjects factor and subject as a random factor and the analysis was conducted in the lme module of R, version 2.8.1 (R Foundation for Statistical Computing, Vienna, Austria), see Barbeau et al. [2013] for details.
Intelligence
We conducted regression analyses with RPM scores and FSIQ, as independent variables to investigate any residual effects of variations in intelligence on perceptual and motor skills. The effects of intelligence, group, and the intelligence × group interactions were explored. We used a complete model to test the effects of intelligence and group and their interactions for the various dependent variables. If the intelligence × group interaction was not significant (P > 0.25), it was removed from the model. Residual normality was tested with the Shapiro‐Wilk test and assumptions (normality, linearity, homoscedasticity) were checked by residual analysis for each model.
Results
Annett Peg Moving Task
When we controlled for intelligence with VIQ, we observed group differences in the dominant hand (DH: F(2,62) = 4.25, P = 0.019), non‐dominant hand (NDH: F(2,61) = 4.52, P = 0.015), and total average conditions (Total: F(2,62) = 5.56, P = 0.006). Planned contrasts revealed that AS‐SOD participants were 772, 876, and 913 ms slower than typical individuals in the DH (P = 0.015), NDH (P = 0.007), and Total (P = 0.003) conditions, respectively. AS‐NoSOD participants were slower than typical individuals in the DH condition only (by 664 ms, P = 0.026; Fig. 1). Controlling for intelligence using the RPM score did not change the overall pattern of the results.
Figure 1.
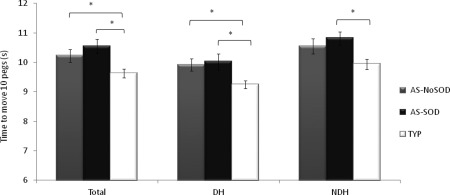
Results of the ANCOVA for the Annett test for the three groups. Average time to move the 10 pegs from one row to another with the dominant hand (DH) or the non‐dominant hand (NDH) and the average of all trials (Total) of the two hands is shown in seconds. *P < 0.05.
The use of 3 SD instead of 2 SD as a cutoff for outliers did not affect the overall trend of the results. Indeed, at 3 SD, group differences were still significant for DH, NDH, and Total when controlling for VIQ, and the AS‐SOD group was significantly slower than the typical group. The AS‐NoSOD group was also slower than the typical group in the NDH condition. Detailed statistical results are shown in Supporting Information Table 1.
Purdue Pegboard
When we controlling for VIQ, we found significant differences between groups in the DH (F(2,63) = 6.37, P = 0.003) and the NDH (F(2,62) = 8.59, P = 0.001) conditions of the Purdue Pegboard Test. Planned contrasts revealed that AS‐NoSOD participants placed on average 9.2% fewer pegs in 30 sec than typical individuals (DH: P = 0.001, NDH: P < 0.001). AS‐SOD individuals tended to be slower than typical individuals in the unimanual conditions but the difference was not significant (DH: P = 0.109, NDH: P = 0.072). The groups also differed in the bimanual condition (BH: F(2,63) = 5.64, P = 0.006), and planned contrasts showed that the AS‐NoSOD group was slower than both the AS‐SOD (6% fewer pegs, P = 0.006) and the typical groups (7.9% fewer pegs, P = 0.003). There was also a significant difference between groups in the Assembly condition (F(2,61) = 5.52, P = 0.015), with planned contrasts showing that the slow performance of the AS‐NoSOD group (7% slower than the AS‐SOD group; P = 0.004) mainly accounted for this difference.
When intelligence was controlled for with RPM IQ score, we found significant differences between groups only for the bimanual conditions of the Purdue, namely the Assembly (F(2,62) = 3.45, P = 0.038) and the BH (F(2,64) = 5.51, P = 0.006) conditions (Fig. 2). Indeed, planned contrasts revealed that AS‐NoSOD participants were 7% slower in the Assembly task than typical (P = 0.015) and AS‐SOD individuals (P = 0.041) and they were also slower than AS‐SOD (P = 0.023) and typical individuals (P = 0.002) in the BH condition.
Figure 2.
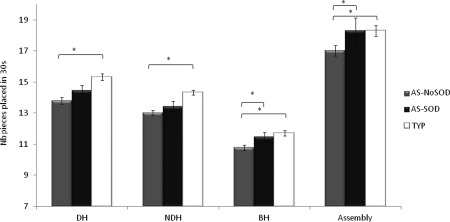
Results of the ANCOVA for the Purdue Pegboard test (DH: dominant Hand, NDH: Non‐Dominant hand, BH: both hands) for the Autism Spectrum with speech onset delay (AS‐SOD) and without (AS‐NoSOD), and typical (TYP) groups. * P < 0.05.
The use of 3 SD as a cutoff for outliers did not affect the overall pattern of the results. When controlling for VIQ, the performance of the groups differed significantly for the unimanual conditions. The AS‐NoSOD group was slower than the typical group, and the AS‐SOD group was also slower than the typical group but for the NDH only. For the bimanual conditions, we observed the same overall trend of group effects: the AS‐NoSOD group was significantly slower than both the AS‐SOD and typical groups. The performance of the AS‐SOD and typical group was similar. See Supporting Information Table 1.
Simple Reaction Time
This visually triggered task is used to isolate movement effects in conditions not requiring visual guidance. We found a difference between groups in performance in this task when controlling for RPM score (F(2,65) = 4.119, P = 0.021). Post hoc tests revealed that this group effect was primarily accounted for by reaction times in AS‐NoSOD participants, which were 31.5 ms faster than in AS‐SOD participants (RPM: P = 0.017; Fig. 3).
Figure 3.
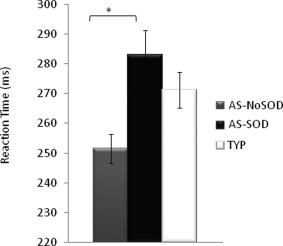
Visually triggered Simple Reaction Time in milliseconds for the AS‐NoSOD, AS‐SOD, and typical (TYP) groups. * P < 0.05.
Inspection Time
AS participants, especially those with speech delay, tended to exhibit shorter inspection times than typical participants (ASP vs. AUT t(59) = 1.28, P = 0.206, AUT vs. TYP t(59) = 1.743, P = 0.086; Fig. 4). The sample from this study overlaps with the sample from Barbeau et al. [2013], in which the difference in inspection time reached significance.
Figure 4.
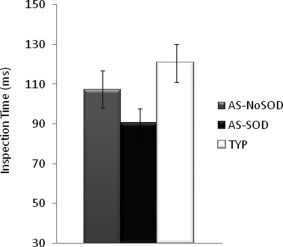
Inspection Time in milliseconds for the AS‐SOD, AS‐NoSOD, and typical (TYP) groups.
Exploratory Regression Analyses
Relationships between intelligence and motor skills
Typical group
In the typical group, RPM score predicted performance in the Purdue Assembly condition. SRT did not predict any of the movement scores in the typical group (see Table 2).
Table 2.
Significant Main Effects and Group Effects or Interactions Are Shown for Regression Analyses With the Purdue or Annett Conditions as Dependent Variables and Intelligence Measures, Simple Reaction Time or Age of First Phrases as Predictive Variables
| Within‐group effects | |||||||
| Main effect | Group effect | Interaction | Typicals | AS‐NoSOD | |||
| Purdue | Assembly | FSIQ | R = 0.451, t(1,43) = 2.604, P = 0.013 | t(1,43) = 1.966, P = 0.056 | R = 0.567, t(1,42)=−2.690 P = 0.010 | R = 0.063 P = 0.763 | R = 0.663, P = 0.003 |
| Within‐group effects | |||||||
| Main effect | Group effect | Interaction | Typicals | AS‐SOD | |||
| Purdue | BH | FSIQ | R = 0.501, t(1,47) = −2.619, P = 0.012 | R = 0.012, P = 0.948 | R = 0.639, P = 0.002 | ||
| DH | SRT | R = 0.466, t(1,47) = 2.000, P = 0.051 | R = 0.006, P = 0.974 | R = 0.505, P = 0.020 | |||
| Annett | DH | SRT | R = 0.537, t(1,47) = 3.145, P = 0.003 | t(1,47) = −2.509, P = 0.016 | R = 0.223, P = 0.244 | R = 0.568, P = 0.007 | |
| Within‐group effects | |||||||
| Main effect | Group effect | Interaction | AS‐NoSOD | AS‐SOD | |||
| Purdue | BH | RPM | R = 0.431, t(1,35) = 2.174, P = 0.037 | t(1,35) = 2.236, P = 0.032 | R = 0.237, P = 0.360 | R = 0.404, P = 0.069 | |
| FSIQ | R = 0.566, t(1,35) = 3.543, P = 0.001 | t(1,35) = 2.689, P = 0.011 | R = 0.565, P = 0.213 | R = 0.639, P = 0.002 | |||
| Assembly | RPM | R = 0.440, t(1,35) = 2.595, P = 0.014 | t(1,35) = 1.790, P = 0.082 | R = 0.491, P = 0.039 | R = 0.356, P = 0.123 | ||
| FSIQ | R = 0.599, t(1,35) = 4.186, P < 0.001 | t(1,35) = 2.243, P = 0.031 | R = 0.636, P = 0.003 | R = 0.533, P = 0.016 | |||
| 1st phrases | R = 0.421, t(1,34) = 2.243, P = 0.032 | R = 0.672, P = 0.002 | R = 0.032, P = 0.922 | ||||
| NDH | SRT | R = 0.476, t(1,34) = −2.952, P = 0.006 | t(1,34) = 2.302, P = 0.028 | R = 0.351, P = 0.167 | R = 0.487, P = 0.029 | ||
| DH | SRT | R = 0.458, t(1,35) = −2.713, P = 0.010 | t(1,35) = 2.458, P = 0.019 | R = 0.251, P = 0.325 | R = 0.505, P = 0.020 | ||
| DH+NDH | 1st phrases | R = 0.476, t(1,33) = 2.759, P = 0.009 | R = 0.703, P = 0.002 | R = 0.061, P = 0.799 | |||
| Annett | DH | SRT | R = 0.500, t(1,35) = 3.397, P = 0.002 | t(1,35) = −1.207, P = 0.235 | R = 0.277, P = 0.279 | R = 0.568, P = 0.007 | |
AS versus Typical groups
The results of the regression analysis differed between the typical and the AS subgroups (Table 2). The performance of AS‐NoSOD participants differed most from that of typical individuals in the Assembly condition of the Purdue Pegboard. The intelligence × group interaction was also significant for FSIQ in this task. Wechsler FSIQ predicted the Assembly score in the AS‐NoSOD group (i.e., the higher the IQ, the faster the motor performance) but not in the typical group.
However, the performance of the AS‐SOD participants differed most from that of the typical group in the bimanual BH condition of the Purdue. The intelligence × group interaction was significant for FSIQ in this condition. Intelligence predicted the performance of AS‐SOD participants in the BH condition but not that of typical individuals. In addition, a slow SRT predicted a slow Purdue DH score in the AS‐SOD group, but not in the typical group.
Effects of speech delay
Within the AS group, RPM score did not predict motor performance in any unimanual conditions of the Annett or Purdue tests. However, RPM score did predict performance in the bimanual conditions of the Purdue Pegboard Test. Among AS individuals with equivalent RPM scores, those with speech delay performed the Purdue BH condition faster than those without speech delay. The relationship between RPM score and the Purdue Assembly condition was similar between groups. Nonetheless, we explored within‐group effects because this variable differed between groups, which showed that a high RPM score predicted a fast performance in the Purdue Assembly task in the AS‐NoSOD group, but not in the AS‐SOD group.
Regarding Wechlser IQ, a high FSIQ predicted a fast performance in the bimanual conditions of the Purdue Pegboard in both groups; however, AS‐SOD participants performed significantly faster than AS‐NoSOD participants with the same FSIQ.
Age of Speech Onset in AS Subgroups
There was an “Age of First Phrases” × group interaction for the unimanual conditions of the Purdue Pegboard. Age of first phrases predicted performance in the unimanual and Assembly conditions in the AS‐NoSOD group, with late speech onset predicting slow motor performance. This effect was not seen in the AS‐SOD group (Table 2).
Simple Reaction Time
In the total AS group, a fast SRT predicted fast motor performance in the Annett DH condition and in the Purdue Pegboard unimanual conditions. AS‐SOD individuals performed the Purdue Pegboard significantly faster than AS‐NoSOD participants with equivalent reaction times. SRT predicted motor performance in AS‐SOD participants but not in AS‐NoSOD participants (Table 2). SRT was not significantly associated with performance in the bimanual conditions of the Purdue Pegboard.
Discussion
Summary of Findings
Here, we used several perceptual and motor tasks measuring key aspects of motor behavior, including gross and fine motor skills, visuo‐spatial integration, dexterity, coordination, and speed, to investigate whether visual processing speed, visually triggered or visually guided movement differs between AS individuals with or without speech onset delay. We also investigated the relationship between motor performance, visual processing speed and intelligence in AS. Our results suggest that AS individuals with speech onset delay perform unimanual motor tasks more slowly than typically developing individuals. AS individuals without speech onset delay showed relatively poor fine motor skills, bimanual coordination, and dexterity. The association between motor skills and intelligence also differed between AS subgroups.
Motor Impairment in AS: Nature and Putative Mechanisms
Overall, our study of AS adolescents and adults is in line with findings of pediatric studies finding both fine and gross motor skill deficits in AS [e.g., Hellendoorn et al., 2015; Lloyd, MacDonald, & Lord, 2011; MacDonald, Lord, & Ulrich, 2014], which suggest that even though some AS symptoms disappear or improve into adulthood, motor deficits might be more stable over time [Staples, MacDonald, & Zimmer, 2012]. AS individuals showed short visual processing time, normal or short SRT, and normal or prolonged visually guided movement time. These findings are consistent with a locus for movement atypicalities at an intermediate stage between visual processing and motor execution. Our findings are consistent with those of Klin et al. [1995] and Szatmari et al. [1990]. They confirm that movement atypicalities in AS‐NoSOD individuals probably involve complex aspects of movements, such as bimanual coordination and dexterity, which are required for the fast manipulation of small objects. The notion that AS‐NoSOD individuals have specific movement atypicalities is consistent with the developmental reorganization of the brain involving region‐specific cortical allocation or resource competition in AS individuals with or without speech delay [for a review see Mottron, Belleville, Rouleau, & Collignon, 2014]. Recently, and in support of this hypothesis, our group reported that in AS‐NoSOD individuals, speech‐like stimuli are processed in a large region overlapping with motor regions. This was not found in AS‐SOD individuals [Samson, Benali, Doyon, Zeffiro, & Mottron, in press]. Another possible interpretation is that both speech and bimanual tasks require a high degree of interhemispheric coordination. AS is often associated with both a thinner than normal corpus callosum [Frazier & Hardan, 2009] and microstructural abnormalities of white matter [Aoki, Abe, Nippashi, & Yamasue, 2013]; therefore, the differences in performance between AS subgroups may result from competition in interhemispheric functional processing capacity between speech and complex limb movements. A final possibility is that symptoms of ADHD, which are more frequent in individuals with DSM‐IV Asperger syndrome [Ghaziuddin, Weidmer‐Mikhail, & Ghaziuddin, 1998; Tani et al., 2006], are involved in this motor impairment. In the current study, only one AS‐SOD and one AS‐NoSOD participant were diagnosed as having comorbid ADHD. The motor performance of these two participants fell within the range of performance of their respective group. Therefore, clinically significant ADHD cannot explain movement atypicalities in the AS‐NoSOD group, but this does not exclude a potential contribution of undiagnosed ADHD.
The AS subgroup with developmental speech delay performed slowly in tasks involving unimanual hand and arm skills, whereas the manipulation of small objects and bimanual coordination were more typical. According to Rinehart et al. [2001], movement atypicalities may be linked to speed of execution or anticipation. This idea is in line with a recent review by Gowen and Hamilton [2013], suggesting that movement atypicalities in autism are related to difficulties in sensorimotor integration and not to movement execution mechanisms per se. Indeed, difficulties in dynamically incorporating visual information during goal‐directed movement may limit the speed at which the movements are carried out to maintain their accuracy. Abnormalities in sensory processing, internal representations [Dewey, Cantell, & Crawford, 2007], and formation and transcoding of spatial representations [Dowell, Mahone, & Mostofsky, 2009] could explain the slow movements we observed in the various tasks; however, the short visual processing time that we observed in both AS groups does not support this interpretation.
Intelligence‐Language‐Motor Skill Relationships
In the group without speech delay, nonverbal intelligence (RPM) predicted the Purdue Assembly score, the task for which these participants showed the poorest performance. By contrast, neither verbal nor nonverbal measures of intelligence were associated with the movement atypicalities observed in the speech delay subgroup. Similarly, later speech onset (albeit in the normal range) was predictive of poor performance in the Purdue Pegboard task in the AS‐NoSOD, but not the AS‐SOD group. Thus, in AS‐SOD individuals, motor performance and intelligence are unrelated, and delayed speech onset is not associated with other cognitive abilities or motor performance. By contrast, motor performance appears to be associated with language abilities and intelligence in AS‐NoSOD. This conclusion is consistent with previous work of our group showing that strong language abilities and high RPM scores coexist in AS individuals without speech onset delay [Barbeau et al., 2013; Soulieres et al., 2011].
Reaction Time and Motor Skills
AS individuals without speech delay showed significantly faster reaction time (SRT), but significantly slower fine motor skills and poorer dexterity and bimanual performance than AS individuals with speech delay. Several factors can influence reaction time, including the speed of neural conduction and general neural integrity [MacDonald, Nyberg, Sandblom, Fischer, & Backman, 2008] and the level of arousal and attention [Davranche, Audiffren, & Denjean, 2006]. The SRT task does not involve a motor response that requires dexterous, agile or coordinated movements of the hand and fingers. This is not the case for the Purdue Pegboard test, which was originally developed to assess both fine finger and goal‐directed movements in industrial workers who require good manual abilities. Thus, AS individuals without speech delay appear to have an intact or highly functioning motor execution system, but they may struggle to incorporate perceptual information during the utilization of more complex and visually guided fine motor movements.
In the AS with speech delay group, the proficiency of unimanual motor skills was predicted by SRT. This result is consistent with the findings of Rinehart et al. [2001] and suggests that the speed at which movements are executed or anticipated could be a limiting factor in goal‐directed movement in autistic people. This cannot result from limitations in the speed of processing visual information, since perceptual processing speed appears quicker in autistic individuals than in both typically developing individuals and individuals with DSM‐IV Asperger syndrome of similar intelligence [Barbeau et al., 2013].
The DSM‐IV Autism‐Asperger Distinction
Our findings suggest that variations in speech onset milestones within the AS are related to limb motor skill development. In AS individuals without speech delay, which broadly coincides with the DSM‐IV definition of Asperger syndrome, fine motor skills, hand movement, and in particular, bimanual coordination, were all impaired. In individuals of typical intelligence with prototypical autism with speech delay, only unimanual motor abilities were impaired. Moreover, a different pattern of relationships between intelligence, language abilities, and movement atypicalities emerges in the two AS subgroups. Motor abilities vary with RPM scores, FSIQ, and language abilities in AS‐NoSOD but are unrelated to intelligence in autism. These observations add to those of cognitive and brain imaging studies [Bonnel et al., 2010; Jones et al., 2009; Sahyoun, Belliveau, Soulieres, Schwartz, & Mody, 2010; Sahyoun, Soulieres, Belliveau, Mottron, & Mody, 2009; Yu, Cheung, Chua, & McAlonan, 2011] demonstrating the effects of developmental speech delay on the heterogeneity of AS phenotype. However, speech delay also aggregates with visuospatial abilities [Barbeau et al., 2013], suggesting that phenotypic heterogeneity in the autistic spectrum is at least partly related to the predominant role of perception in AS‐SOD and of language in AS‐No‐SOD in the cognitive architecture of AS individuals [see Mottron et al., 2014 for a model, and Samson et al. [in press] for a fMRI demonstration]. Whereas DSM‐IV uses a subgrouping strategy to account for autistic spectrum heterogeneity, the DSM‐5 uses clinical specifiers, thereby allowing an indefinite number of possible combinations of specifier values. Whether the heterogeneity of the AS is best characterized as combinations of continuous physiological and behavioral dimensions, or by distinct phenotypic subgroups, remains an open issue. Nonetheless, the current findings suggest that the most frequent clusters between different specifier should be integrated in the clinical knowledge.
Acknowledgments
This work was supported by an operating grant from the Canadian Institutes of Health Research (CIHR, No. MOP‐84243) awarded to L. Mottron, and scholarships from the CIHR awarded to E.B. Barbeau and A.‐A. S. Meilleur. The authors also want to thank the participants for their invaluable contribution to this project. The authors report no potential conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web‐site
Supplementary Table 1. ANCOVAs with VIQ as a covariate. Group effects are displayed as well as planned contrasts comparing the Autism Spectrum with speech onset delay (AS‐SOD) and without (AS‐NoSOD) and typical (TYP) groups for the Annett dominant hand (DH), non‐dominant hand (NDH) and average (Total) conditions and the Purdue dominant hand (DH), non‐dominant hand (NDH), both hands (BH) and Assembly conditions.
References
- Annett, Marian. (2002). Handedness and brain asymmetry: The right shift theory. Philadelphia, PA: Psychology Press. [Google Scholar]
- Aoki, Y. , Abe, O. , Nippashi, Y. , & Yamasue, H. (2013). Comparison of white matter integrity between autism spectrum disorder subjects and typically developing individuals: A meta‐analysis of diffusion tensor imaging tractography studies. Molecular Autism, 4(1), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA . (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- APA . (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Barbeau, E.B. , Soulieres, I. , Dawson, M. , Zeffiro, T.A. , & Mottron, L. (2013). The level and nature of autistic intelligence III: Inspection time. Journal of Abnormal Psychology, 122(1), 295–301. [DOI] [PubMed] [Google Scholar]
- Behere, A. , Shahani, L. , Noggle, C.A. , & Dean, R. (2012). Motor functioning in autistic spectrum disorders: A preliminary analysis. The Journal of Neuropsychiatry and Clinical Neurosciences, 24(1), 87–94. [DOI] [PubMed] [Google Scholar]
- Bonnel, A. , McAdams, S. , Smith, B. , Berthiaume, C. , Bertone, A. , Ciocca, V. , et al. (2010). Enhanced pure‐tone pitch discrimination among persons with autism but not Asperger syndrome. Neuropsychologia, 48(9), 2465–2475. [DOI] [PubMed] [Google Scholar]
- Brisson, J. , Warreyn, P. , Serres, J. , Foussier, S. , & Adrien, J.L. (2012). Motor anticipation failure in infants with autism: A retrospective analysis of feeding situations. Autism, 16, 420–429. [DOI] [PubMed] [Google Scholar]
- Davranche, K. , Audiffren, M. , & Denjean, A. (2006). A distributional analysis of the effect of physical exercise on a choice reaction time task. Journal of Sports Sciences, 24(3), 323–329. [DOI] [PubMed] [Google Scholar]
- Dawson, M. , Soulieres, I. , Gernsbacher, M.A. , & Mottron, L. (2007). The level and nature of autistic intelligence. Psychological Science, 18(8), 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker, S.L. , Englund, J.A. , Carboni, J.A. , & Brooks, J.H. (2011). Cognitive and developmental influences in visual‐motor integration skills in young children. Psychological Assessment, 23(4), 1010–1016. [DOI] [PubMed] [Google Scholar]
- Dewey, D. , Cantell, M. , & Crawford, S.G. (2007). Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society, 13(2), 246–256. [DOI] [PubMed] [Google Scholar]
- Dowd, A.M. , McGinley, J.L. , Taffe, J.R. , & Rinehart, N.J. (2011). Do planning and visual integration difficulties underpin motor dysfunction in autism? A kinematic study of young children with autism. Journal of Autism and Developmental Disorders, 42, 1539–1548. [DOI] [PubMed] [Google Scholar]
- Dowell, L.R. , Mahone, E.M. , & Mostofsky, S.H. (2009). Associations of postural knowledge and basic motor skill with dyspraxia in autism: Implication for abnormalities in distributed connectivity and motor learning. Neuropsychology, 23(5), 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziuk, M.A. , Gidley Larson, J.C. , Apostu, A. , Mahone, E.M. , Denckla, M.B. , & Mostofsky, S.H. (2007). Dyspraxia in autism: Association with motor, social, and communicative deficits. Developmental Medicine and Child Neurology, 49(10), 734–739. [DOI] [PubMed] [Google Scholar]
- Forti, S. , Valli, A. , Perego, P. , Nobile, M. , Crippa, M. , & Molteni, M. (2011). Motor planning and control in autism. A kinematic analysis of preschool children. Research in Autism Spectrum Disorders, 5(2), 834–842. [Google Scholar]
- Fournier, K.A. , Hass, C.J. , Naik, S.K. , Lodha, N. , & Cauraugh, J.H. (2010). Motor coordination in autism spectrum disorders: A synthesis and meta‐analysis. Journal of Autism and Developmental Disorders, 40(10), 1227–1240. [DOI] [PubMed] [Google Scholar]
- Frazier, T.W. , & Hardan, A.Y. (2009). A meta‐analysis of the corpus callosum in autism. Biological Psychiatry, 66(10), 935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepner, B. , & Feron, F. (2009). Autism: A world changing too fast for a mis‐wired brain? Neuroscience and Biobehavioral Reviews, 33(8), 1227–1242. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin, M. , Weidmer‐Mikhail, E. , & Ghaziuddin, N. (1998). Comorbidity of Asperger syndrome: A preliminary report. Journal of Intellectual Disability Research, 42 (Pt 4), 279–283. [DOI] [PubMed] [Google Scholar]
- Gillberg, C. (1998). Asperger syndrome and high‐functioning autism. British Journal of Psychiatry, 172, 200–209. [DOI] [PubMed] [Google Scholar]
- Glazebrook, C.M. , Elliott, D. , & Lyons, J. (2006). A kinematic analysis of how young adults with and without autism plan and control goal‐directed movements. Motor Control, 10(3), 244–264. [DOI] [PubMed] [Google Scholar]
- Gowen, E. , & Hamilton, A. (2013). Motor abilities in autism: A review using a computational context. Journal of Autism and Developmental Disorders, 43(2), 323–344. [DOI] [PubMed] [Google Scholar]
- Green, D. , Charman, T. , Pickles, A. , Chandler, S. , Loucas, T. , Simonoff, E. , et al. (2009). Impairment in movement skills of children with autistic spectrum disorders. Developmental Medicine and Child Neurology, 51(4), 311–316. [DOI] [PubMed] [Google Scholar]
- Hartman, E. , Houwen, S. , Scherder, E. , & Visscher, C. (2010). On the relationship between motor performance and executive functioning in children with intellectual disabilities. Journal of Intellectual Disability Research, 54(5), 468–477. [DOI] [PubMed] [Google Scholar]
- Hellendoorn, A. , Wijnroks, L. , van Daalen, E. , Dietz, C. , Buitelaar, J.K. , & Leseman, P. (2015). Motor functioning, exploration, visuospatial cognition and language development in preschool children with autism. Research in Developmental Disorders, 39, 32–42. [DOI] [PubMed] [Google Scholar]
- Hughes, C. (1996). Brief report: Planning problems in autism at the level of motor control. Journal of Autism and Developmental Disorders, 26(1), 99–107. [DOI] [PubMed] [Google Scholar]
- Jansiewicz, E.M. , Goldberg, M.C. , Newschaffer, C.J. , Denckla, M.B. , Landa, R. , & Mostofsky, S.H. (2006). Motor signs distinguish children with high functioning autism and Asperger's syndrome from controls. Journal of Autism and Developmental Disorders, 36(5), 613–621. [DOI] [PubMed] [Google Scholar]
- Johnson, B.P. , Rinehart, N.J. , White, O. , Millist, L. , & Fielding, J. (2013). Saccade adaptation in autism and Asperger's disorder. Neuroscience, 243, 76–87. [DOI] [PubMed] [Google Scholar]
- Jones, C.R. , Happe, F. , Baird, G. , Simonoff, E. , Marsden, A.J. , Tregay, J. , et al. (2009). Auditory discrimination and auditory sensory behaviours in autism spectrum disorders. Neuropsychologia, 47(13), 2850–2858. [DOI] [PubMed] [Google Scholar]
- Klin, A. , Volkmar, F.R. , Sparrow, S.S. , Cicchetti, D.V. , & Rourke, B.P. (1995). Validity and neuropsychological characterization of Asperger syndrome: Convergence with nonverbal learning disabilities syndrome. Journal of Child Psychology and Psychiatry, 36(7), 1127–1140. [DOI] [PubMed] [Google Scholar]
- Landa, R. , & Garrett‐Mayer, E. (2006). Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry, 47(6), 629–638. [DOI] [PubMed] [Google Scholar]
- Linkenauger, S.A. , Lerner, M.D. , Ramenzoni, V.C. , & Proffitt, D.R. (2012). A perceptual‐motor deficit predicts social and communicative impairments in individuals with autism spectrum disorders. Autism Research, 5(5), 352–362. [DOI] [PubMed] [Google Scholar]
- Lloyd, M. , MacDonald, M. , & Lord, C. (2011). Motor skills of toddlers with autism spectrum disorders. Autism, 17(2), 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord, C. , Risi, S. , Lambrecht, L , Cook, E.H. Jr. , Leventhal, B.L. , DiLavore, P.C. , et al. (2000). The autism diagnostic observation schedule‐generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223. [PubMed] [Google Scholar]
- Lord, C. , Rutter, M. , & Le Couteur, A. (1994). Autism Diagnostic Interview‐Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. [DOI] [PubMed] [Google Scholar]
- MacDonald, M. , Lord, C. , & Ulrich, D. (2014). Motor skills and calibrated autism severity in young children with autism spectrum disorder. Adapted Physical Activity Quarterly, 31, 95–105. [DOI] [PubMed] [Google Scholar]
- MacDonald, S.W. , Nyberg, L. , Sandblom, J. , Fischer, H. , & Backman, L. (2008). Increased response‐time variability is associated with reduced inferior parietal activation during episodic recognition in aging. Journal of Cognitive Neuroscience, 20(5), 779–786. [DOI] [PubMed] [Google Scholar]
- Macintosh, K.E. , & Dissanayake, C. (2004). Annotation: The similarities and differences between autistic disorder and Asperger's disorder: A review of the empirical evidence. Journal of Child Psychology and Psychiatry, 45(3), 421–434. [DOI] [PubMed] [Google Scholar]
- Manjiviona, J. , & Prior, M. (1995). Comparison of Asperger syndrome and high‐functioning autistic children on a test of motor impairment. Journal of Autism and Developmental Disorders, 25(1), 23–39. [DOI] [PubMed] [Google Scholar]
- Mayes, S.D. , & Calhoun, S.L. (2007). Learning, attention, writing, and processing speed in typical children and children with ADHD, autism, anxiety, depression, and oppositional‐defiant disorder. Child Neuropsychology, 13(6), 469–493. [DOI] [PubMed] [Google Scholar]
- Memisevic, H. , & Sinanovic, O. (2012). Predictors of visual‐motor integration in children with intellectual disability. International Journal of Rehabilitation Research, 35(4), 372–374. [DOI] [PubMed] [Google Scholar]
- Mostofsky, S.H. , Burgess, M.P. , & Gidley Larson, J.C. (2007). Increased motor cortex white matter volume predicts motor impairment in autism. Brain, 130(Pt 8), 2117–2122. [DOI] [PubMed] [Google Scholar]
- Mottron, L. , Belleville, S. , Rouleau, G.A. , & Collignon, O. (2014). Linking neocortical, cognitive, and genetic variability in autism with alterations of brain plasticity: The Trigger‐Threshold‐Target model. Neuroscience and Biobehavioral Review, 47, 735–752. [DOI] [PubMed] [Google Scholar]
- Nayate, A. , Tonge, B.J. , Bradshaw, J.L. , McGinley, J.L. , Iansek, R. , & Rinehart, N.J. (2012). Differentiation of high‐functioning autism and Asperger's disorder based on neuromotor behaviour. Journal of Autism and Developmental Disorders, 42(5), 707–717. [DOI] [PubMed] [Google Scholar]
- Noterdaeme, M. , Wriedt, E. , & Hohne, C. (2010). Asperger's syndrome and high‐functioning autism: Language, motor and cognitive profiles. European Child & Adolescent Psychiatry, 19(6), 475–481. [DOI] [PubMed] [Google Scholar]
- Oldfield, R.C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Papadopoulos, N. , McGinley, J. , Tonge, B. , Bradshaw, J. , Saunders, K. , Murphy, A. , et al. (2012). Motor proficiency and emotional/behavioural disturbance in autism and Asperger's disorder: Another piece of the neurological puzzle? Autism, 16(6), 627–640. [DOI] [PubMed] [Google Scholar]
- Rinehart, N.J. , Bellgrove, M.A. , Tonge, B.J. , Brereton, A.V. , Howells‐Rankin, D. , & Bradshaw, J. L. (2006). An examination of movement kinematics in young people with high‐functioning autism and Asperger's disorder: Further evidence for a motor planning deficit. Journal of Autism and Developmental Disorders, 36(6), 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart, N.J. , Bradshaw, J.L. , Brereton, A.V. , & Tonge, B.J. (2001). Movement preparation in high‐functioning autism and Asperger disorder: A serial choice reaction time task involving motor reprogramming. Journal of Autism and Developmental Disorders, 31(1), 79–88. [DOI] [PubMed] [Google Scholar]
- Sahyoun, C.P. , Belliveau, J.W. , Soulieres, I. , Schwartz, S. , & Mody, M. (2010). Neuroimaging of the functional and structural networks underlying visuospatial vs. linguistic reasoning in high‐functioning autism. Neuropsychologia, 48(1), 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun, C.P. , Soulieres, I. , Belliveau, J.W. , Mottron, L. , & Mody, M. (2009). Cognitive differences in pictorial reasoning between high‐functioning autism and Asperger's syndrome. Journal of Autism and Developmental Disorders, 39(7), 1014–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson, F. , Benali, H. , Doyon, J. , Zeffiro, T.A. & Mottron, L. Speech acquisition predicts regions of enhanced cortical response to auditory stimulation in autism spectrum individuals. Journal of Psychiatric Research, in press. [DOI] [PubMed] [Google Scholar]
- Smits‐Engelsman, B. , & Hill, E.L. (2012). The relationship between motor coordination and intelligence across the IQ range. Pediatrics, 130(4), e950–e956. [DOI] [PubMed] [Google Scholar]
- Soper, H.V. , Satz, P. , Orsini, D.L. , Henry, R.R. , Zvi, J.C. , & Schulman, M. (1986). Handedness patterns in autism suggest subtypes. Journal of Autism and Developmental Disorders, 16(2), 155‐–167. [DOI] [PubMed] [Google Scholar]
- Soulieres, I. , Dawson, M. , Gernsbacher, M.A. , & Mottron, L. (2011). The level and nature of autistic intelligence II: What about Asperger syndrome? PLoS One, 6(9), e25372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley‐Cary, C. , Rinehart, N. , Tonge, B. , White, O. , & Fielding, J. (2011). Greater disruption to control of voluntary saccades in autistic disorder than Asperger's disorder: Evidence for greater cerebellar involvement in autism? Cerebellum, 10(1), 70–80. [DOI] [PubMed] [Google Scholar]
- Staples, K. , MacDonald, M. , & Zimmer, C. (2012). Assessment of motor behavior among children and adolescents with autism spectrum disorder. International Review of Research in Developmental Disabilities, 42, 179–214. [Google Scholar]
- Stoit, A.M. , van Schie, H.T. , Slaats‐Willemse, D.I. , & Buitelaar, J.K. (2013). Grasping motor impairments in autism: Not action planning but movement execution is deficient. Journal of Autism and Developmental Disorders, 43(12), 2793–2806. [DOI] [PubMed] [Google Scholar]
- Szatmari, P. , Tuff, L. , Finlayson, M.A. , & Bartolucci, G. (1990). Asperger's syndrome and autism: Neurocognitive aspects. Journal of the American Academy of Child and Adolescent Psychiatry, 29(1), 130–136. [DOI] [PubMed] [Google Scholar]
- Tani, P. , Lindberg, N. , Appelberg, B. , Nieminen‐von Wendt, T. , von Wendt, L. , & Porkka‐Heiskanen, T. (2006). Childhood inattention and hyperactivity symptoms self‐reported by adults with Asperger syndrome. Psychopathology, 39(1), 49–54. [DOI] [PubMed] [Google Scholar]
- Teitelbaum, O. , Benton, T. , Shah, P.K. , Prince, A. , Kelly, J.L. , & Teitelbaum, P. (2004). Eshkol‐Wachman movement notation in diagnosis: The early detection of Asperger's syndrome. Proceedings of the National Academy of Sciences of the United States of America, 101(32), 11909–11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorp, M. , Houwen, S. , Hartman, E. , & Visscher, C. (2011). Are gross motor skills and sports participation related in children with intellectual disabilities? Research in Developmental Disabilities, 32(3), 1147–1153. [DOI] [PubMed] [Google Scholar]
- WHO . (1992). The ICD‐10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Geneva: World Health Orgnization. [Google Scholar]
- Whyatt, C.P. , & Craig, C.M. (2012). Motor skills in children aged 7‐10 years, diagnosed with autism spectrum disorder. Journal of Autism and Developmental Disorders, 42, 1799–1809. [DOI] [PubMed] [Google Scholar]
- Yu, K.K. , Cheung, C. , Chua, S.E. , & McAlonan, G.M. (2011). Can Asperger syndrome be distinguished from autism? An anatomic likelihood meta‐analysis of MRI studies. Journal of Psychiatry & Neuroscience, 36(6), 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's web‐site
Supplementary Table 1. ANCOVAs with VIQ as a covariate. Group effects are displayed as well as planned contrasts comparing the Autism Spectrum with speech onset delay (AS‐SOD) and without (AS‐NoSOD) and typical (TYP) groups for the Annett dominant hand (DH), non‐dominant hand (NDH) and average (Total) conditions and the Purdue dominant hand (DH), non‐dominant hand (NDH), both hands (BH) and Assembly conditions.


