Figure 1. Identification of oridonin as a novel acetyltransferase inhibitor.
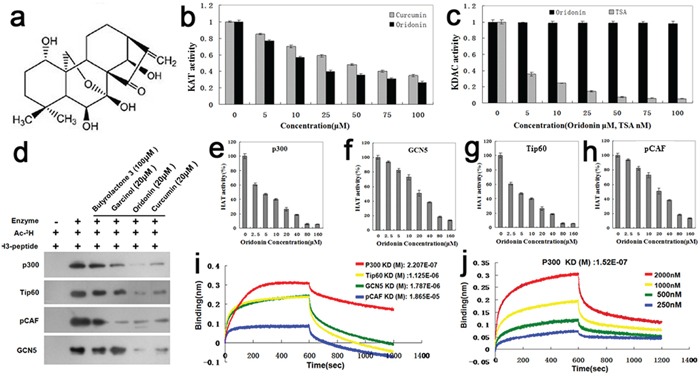
a. Structure of oridonin: oridonin belongs to the ene-kaurane diterpenoids. b. Comparison of the KATi activity of oridonin with that of a known KAT inhibitor curcumin by using the KAT Activity Colorimetric Assay Kit. Bars represent means±S.E.M. (n=3). c. Comparison of the KDACi activity of oridonin with that of a known KDAC inhibitor TSA by using the KDAC Inhibitor Drug Screening Kit (Fluorometric) (BioVision). Bars represent means±S.E.M. (n=3). d. The in vitro inhibitory effect of oridonin on acetylation of histone 3 was validated by autoradiography. The KATi effects of Oridonin on p300, Tip60, Pcaf and GCN5 were compared with those of established KATis such asbutyrolactone 3, curcumin and garcinol. (e-h) Assays of the KATi activities of Oridonin on P300 e. GCN5 f. Tip60 g. and pCAF h. Bars represent means±S.E.M. (n=4). i. The equilibrium dissociation constants between oridonin and the four acetyltransferases (P300, GCN5, Tip60 and pCAF) were measured by the ForteBio Octet RED96 system. j. The equilibrium dissociation constant kD (M) between oridonin and P300 of different concentrations (250, 500, 1000 and 2000 nM).
