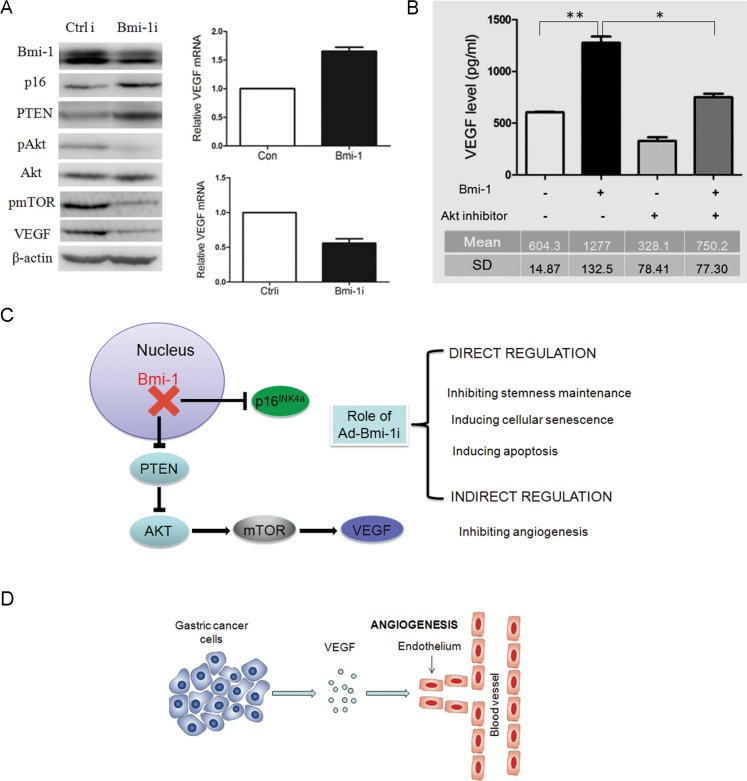
Figure 5. Knockdown of Bmi-1 by Ad-Bmi-1i transfection results in the downregulation of VEGF via the PTEN/AKT/mTOR pathway.
(A) Knockdown of Bmi-1 leads to upregulation of PTEN proteins, and reduction in pAKT (phosphorylated AKT), downregulation of p-mTOR and downregulation of VEGF expression as determined by Western blot analysis. β-actin is an internal control (left panel). Overexpression of Bmi-1 leads to increase of VEGF mRNA expression as determined by qRT-PCR analysis; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is an internal control (upper right panel). Transient transfection of Bmi-1 short hairpin RNA (shRNA) in MKN45 cells results in decrease of VEGF mRNA expression as determined by qRT-PCR. GAPDH is an internal control (upper right panel). (B) AKT inhibitor (cell signaling Wortmannin #9951, 0.25 uM) can partially reverse the increased VEGF secretion induced by Bmi-1 overexpression in gastric cancers. The VEGF concentrations in fixed 2 ml supernatant were detected by ELISA assay in SGC-7901 cells expressing Bmi-1 or Bmi-1 together with AKT inhibitor (mean ± SD). (C) The two molecular pathways (p16 and PTEN/AKT/mTOR/VEGR) of Bmi-1 shRNA in GC. Ad-Bmi-1i induces upregulation of p16 expression, eventually leading to the direct killing or inhibitory role (blockage of stemness maintenance, inducing cellular senescence or apoptosis) and indirect killing or inhibitory role (decreasing formation of blood vessels surrounding the tumor) for gastric cancer. (D) The regulation of Bmi-1 for angiogenesis in gastric cancer cells. Bmi-1 overexpression can increase the secretion of VEGF in SGC-7901 cells, and subsequently promote angiogenesis surrounding the tumor, further inducing the progression of cancer via the indirect or direct role. *p < 0.05, **p < 0.01.