Figure 1. Lysine 392 on SUV39H2 is automethylated.
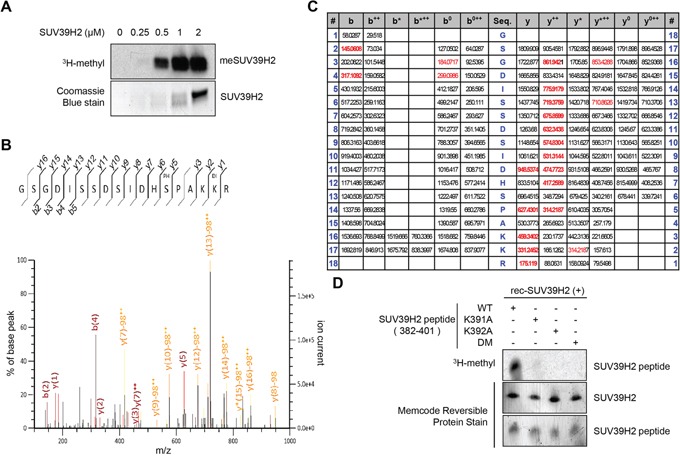
A. The automethylation intensities of SUV39H2 were proportional to the amounts of SUV39H2 protein. An in vitro methyltransferase assay was performed by using purified His-tagged SUV39H2 recombinant proteins. Methylated SUV39H2 was detected by autoradiography. Amounts of loading proteins were confirmed by staining with Coomassie Brilliant Blue. B. The MS-MS spectrum corresponding to the dimethylated and phosphorylated SUV39H2 376–393 peptide. The 28 Da increase of the lysine 392 residue was detected. C. MS/MS spectra of the SUV39H2 peptide corresponding to 376-393 amino acids. LC-MS/MS analysis showed methylation of SUV39H2 at lysine 392. Theoretical values of MS fragments are summarized. D. An in vitro methyltransferase assay indicated that wild-type SUV39H2 peptide (WT, 382-401 amino acids) was methylated by His-tagged SUV39H2 recombinant proteins but not lysine 391, lysine 392, or both lysines 391 and 392-substituted SUV39H2 peptide (K391A/K392A/DM). Each SUV39H2 peptide (WT, K391A, K392A or K391A/K392A/DM) was mixed with His-tagged recombinant protein in 50 mM Tris-HCl (pH8.8) buffer with 1.0 μCi/ml S-adenosyl-L-[mehyl-3H]-methionine for 2 hours at 30°C. After boiling in the sample buffer, the samples were subjected to SDS-PAGE, and visualized by autoradiography. Amounts of loading proteins were evaluated by staining the MemCode™ Reversible Protein Stain (Thermo Fisher Scientific).
