Abstract
tRNAs are transcribed as precursors containing 5′ leader and 3′ extensions that are removed by a series of posttranscriptional processing reactions to yield functional mature tRNAs. Here, we examined the maturation pathway of tRNAMet in Trypanosoma brucei, an early divergent unicellular eukaryote. We identified an approximately 300-kDa complex located in the nucleus of T. brucei that is required for trimming the 5′ leader of initiator tRNAMet precursors. One of the subunits of the complex (T. brucei MT40 [TbMT40]) is a putative methyltransferase and a homolog of Saccharomyces cerevisiae Gcd14, which is essential for 1-methyladenosine modification in tRNAs. Down-regulation of TbMT40 by RNA interference resulted in the accumulation of precursor initiator tRNAMet containing 5′ extensions but processed 3′ ends. In addition, immunoprecipitations with anti-La antibodies revealed initiator tRNAMet molecules with 5′ and 3′ extensions in TbMT40-silenced cells, albeit at a much lower level. Interestingly, silencing of TbMT40, as well as of TbMT53, a second subunit of the complex, led to an increase in the levels of mature elongator tRNAMet. Taken together, our data provide a glance at the maturation of tRNAs in parasitic protozoa and suggest that at least for initiator tRNAMet, 3′ trimming precedes 5′ processing.
The primary transcripts of tRNAs undergo a series of posttranscriptional maturation steps, including splicing, trimming at the 5′ and 3′ ends, and modifications of the base or ribose moiety. Pre-tRNAs containing introns are processed by a specific RNA splicing mechanism involving cleavage and religation. Maturation of the tRNA 5′ end is the task of endonuclease RNase P, a well-studied enzyme in both prokaryotes and eukaryotes (7). 3′ trimming is not as well understood and can involve endonucleolytic as well as exonucleolytic cleavage, with most of the eukaryotic systems analyzed so far using endonucleolytic processing (16). Of note is that endonucleolytic 3′ processing in Saccharomyces cerevisiae is stimulated by the La protein (29).
Modified nucleosides have been identified in all cellular RNAs, with tRNAs representing the greatest number and variety of modifications. In particular, 1-methyladenosine (m1A) at position 58 of the TΨC loop has been found in tRNAs from all three kingdoms (4). Although this modification is widespread in S. cerevisiae tRNAs (24), elimination of all m1A modifications specifically reduced the processing and stability of initiator methionyl-tRNA (tRNAiMet) and had little effect on other tRNAs, suggesting a unique role for this methylation in the maturation pathway of tRNAiMet (2). This modification is introduced in the tRNAs by an essential complex containing Gcd10 protein (Gcd10p) and Gcd14p (2, 3), which were originally defined as proteins required for the initiation of protein synthesis and translational repression of GCN4 mRNA (10). What is intriguing about their involvement in m1A formation is that this represents the first two-component methyltransferase complex (3), since all well-characterized tRNA methyltransferases are single-subunit enzymes. Although Gcd14p has signature motifs of methyltransferases, it is not capable of generating the m1A modification and of binding to tRNAiMet, unless it forms a complex with Gcd10p (3). These observations are consistent with a role for Gcd10p in the binding of the tRNA substrate. In spite of the apparent importance of m1A, eukaryotic studies of the function of the modified residue or of the enzymes that catalyze this modification (Gcd10p and Gcd14p) have so far been limited to S. cerevisiae.
The maturation of tRNAs in members of the Kinetoplastidae family, including Trypanosoma brucei, is not a subject of intense research. This fact is somewhat surprising, since these organisms have unique features: the mitochondrion does not encode tRNAs and, thus, a complete set of tRNAs must be imported from the cytosol (20). In particular, there is evidence to suggest that some tRNAs are imported as precursors before they are processed to mature tRNAs (13). Another interesting twist in this scenario is that although tRNAiMet and elongator tRNAMet are found in the cytosol, only elongator tRNAMet is imported into mitochondria (25). Since little is known about the rules dictating mitochondrial import of tRNAs, it remains a challenge to understand why one cytosolic tRNA is imported in T. brucei and another is not.
In this study, we examined the maturation pathway of tRNAMet by silencing T. brucei homologs of Gcd10p and Gcd14p, which are essential for tRNAiMet maturation in S. cerevisiae (2, 3).
MATERIALS AND METHODS
Plasmid constructs, transfections, and cell lines.
To generate the T. brucei MT40 (TbMT40) RNA interference (RNAi) cell line, a 900-bp fragment (nucleotides [nt] 170 to 1070) of the TbMT40 translated region was assembled as two inverted repeats separated by a stuffer fragment and inserted downstream of a tetracycline (TET)-inducible promoter from the procyclic acidic repetitive protein genes (26). Similarly, the MT53 RNAi construct was generated with a 498-bp fragment from within the TbMT53 coding region (nt 245 to 743). Both constructs were linearized with EcoRV for integration at the ribosomal DNA nontranscribed spacer region of strain 29.13.6, expressing the TET repressor and T7 RNA polymerase (27). Transformed cells were selected in the presence of 2.5 μg of phleomycin/ml and cloned by limiting dilution.
A PCR-based method (22) was used to establish a cell line in which TbMT40 and TbMT53 were epitope tagged at their N termini with BB2 and hemagglutinin (HA), respectively. The approach entailed PCR amplification of a reporter cassette with two primers containing flanking sequences specific to the target gene. The PCR product was then transfected into T. brucei procyclic cells, and homologous recombinants that carry the deleted or tagged target gene were identified. Similarly, we established a cell line expressing tandem affinity purification (TAP)-tagged TbMT40. The TAP tag, consisting of two protein A immunoglobulin G binding domains, a tobacco etch virus protease cleavage site and a calmodulin binding domain, was inserted at the N terminus of TbMT40 in a strain where the second allele was replaced by the blasti-cidin resistance marker.
RNAi induction, RNA isolation, and Northern analysis.
To induce expression of double-stranded RNA, cells were diluted to 106 cells/ml each day and TET was added to a final concentration of 10 μg/ml. Total RNA was prepared by lysing cells with Trizol reagent. Ten micrograms of total RNA per lane was fractionated on a 15% polyacrylamide-7 M urea gel and electrotransferred onto a nylon membrane (Hybond; Amersham Biosciences). tRNA>iMet and elongator tRNAMet were detected by hybridization at 55°C with radiolabeled oligonucleotides in a solution containing 6× SSPE (1.08 M NaCl, 60 mM NaPO4 [pH 7.7], 6 mM EDTA), 5× Denhardt's solution (Ficoll, polyvinylpyrrolidone, and bovine serum albumin; 0.1% [wt/vol] each), and 0.5% sodium dodecyl sulfate (SDS). Filters were washed two times for 5 min each in 2× SSPE-0.5% SDS at 55°C. The detection and quantitation of hybridization signals were done with a Cyclone phosphorimager (Packard) and with OptiQuant software, respectively.
The following oligonucleotides were used as probes for Northern blot analysis: 5′-GTTGGTTTCGATCCAACG-3′, which is complementary to nt 47 to 64 of tRNAiMet; 5′-GCGCCACGGTGCTTCGGGGACCCGGC-3′, which is complementary to nt −13 to +13 of the tRNAiMet; and 5′-GTGAGGCTCGAACTCACG-3′, which is complementary to nt 48 to 65 of elongator tRNAMet.
Immunoprecipitations and Western blot analysis.
Cell extracts were prepared by breaking the cells in a solution of 10 mM HEPES [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 1 mM dithiothreitol, and 10 μg of leupeptin/ml with a French press, applying a pressure of 6,000 lb/in2. The resulting extract was centrifuged at 5,000 × g for 15 min, and a 1/10 volume of 0.3 M HEPES (pH 7.9)-1.3 M KCl-30 mM MgCl2 was added to the supernatant. Following centrifugation at 100,000 × g for 45 min, the final supernatant (S-100) was used for immunoprecipitations. Anti-BB2 or anti-HA antibodies were bound to protein G-Sepharose and protein A-Sepharose beads, respectively. After being washed with NET-2 buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.05% Nonidet P-40), the beads were mixed with the S-100 and incubated for 4 h at 4°C. The beads were then washed eight times with NET-2 and an equivalent amount of the supernatant, and the immunoprecipitate was analyzed by SDS-PAGE followed by Western blotting as described previously (23).
La immunoprecipitations were performed with anti-La polyclonal antibodies (15) and whole-cell extracts prepared from TbMT40 RNAi cells that were induced with TET for 72 h. Following immunoprecipitations, RNA was extracted with Trizol reagent and analyzed by Northern blotting.
Other procedures.
Cells were processed for double-label indirect immunofluorescence as described previously (17). Cell fractionation and gel filtration with a Superdex-200 column (Amersham Biosciences) were carried out as described previously (6).
RESULTS
GCD10 and GCD14 homologs in trypanosomatids.
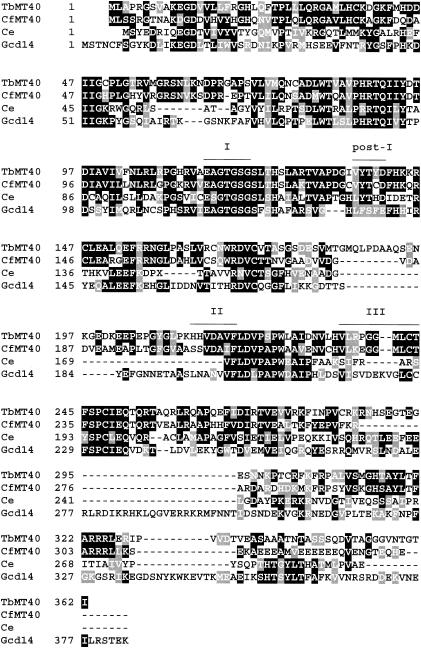
GCD10- and GCD14-related genes were originally identified in trypanosomatids through direct mass spectrometric sequencing of two gel-separated Crithidia fasciculata proteins present in a fraction highly enriched for RNA methyltransferases (C. Tschudi, unpublished data). The two genes were named the C. fasciculata MT40 (CfMT40) and CfMT51 genes, according to their predicted molecular weights. A BLAST search (1) of the nonredundant translated GenBank database at that time with the primary amino acid sequence of CfMT40 showed the highest identity scores with a putative open reading frame in Caenorhabditis elegans (42% identity; accession number Z81587), a hypothetical Schizosaccharomyces pombe protein (40% identity; accession number Z98763), and the S. cerevisiae Gcd14 protein (38% identity; accession number CAA90863). In addition, homologs were found in various eukaryotic organisms by searching the EST, HTGS, and GSS databases, thus defining a cluster of closely related proteins present in trypanosomes, fungi, nematodes, insects, and mammals. Through these searches, it also became apparent that CfMT40 was related to S-adenosyl-l-methionine (SAM)-dependent methyltransferases (Fig. 1).
FIG. 1.
Clustal alignment of TbMT40 (Sanger number TRYP_xi1031a02.p1k_70), CfMT40, the C. elegans MT40 homolog (Ce) (accession number Z81587), and S. cerevisiae GCD14 (Gcd14) (accession number CAA90863). Motifs characteristic of SAM-dependent methyltransferases are indicated above the sequences.
A comparison of the inferred CfMT51 protein sequence with those in GenBank revealed no convincing similarities to methyltransferases. However, similarly to CfMT40 described above, CfMT51 identified a group of related proteins in many eukaryotic organisms. Whereas most of these proteins did not have a known function, the S. cerevisiae protein turned out to be Gcd10p (9), which was originally isolated in the same genetic screen as Gcd14p (10).
Subsequent to the above searches, we identified GCD10- and GCD14-related genes in a variety of trypanosomatids, including T. brucei, which was used as an experimental system for the studies described in this paper. At the amino acid level, TbMT40 and TbMT53 have 35 and 23% identity (50 and 46% similarity) with Gcd14p and Gcd10p, respectively. Like yeast Gcd14p (5), TbMT40 has signature motifs of SAM-dependent methyltransferases (11). Motif I and post-I, comprising part of the SAM binding pocket, matched amino acids 115 to 121 and 137 to 141, respectively (Fig. 1). Two other regions, termed motifs II and III, are present in SAM-dependent methyltransferases, and they were assigned to amino acids 213 to 220 and 235 to 244, respectively.
TbMT40 silencing affects tRNAMet processing.
To begin to address the functions of TbMT40 and TbMT53, both of their mRNAs were down-regulated by RNAi. For each gene, we generated a construct containing two inverted repeats of a portion of the coding region separated by a stuffer fragment. The cassette is under the control of the TET-inducible promoter from the procyclic acidic repetitive protein genes, which will drive expression of hairpin double-stranded RNA. The construct was integrated at the ribosomal DNA nontranscribed spacer, and stable clonal cell lines were established. A Northern blot analysis of RNA isolated from cells induced with TET revealed that the TbMT40 mRNA was reduced to undetectable levels after 1 day of induction and remained at that level for an additional 4 days of induction (Fig. 2), whereas there was a residual amount of TbMT53 mRNA during a similar induction period (data not shown). This down-regulation of the mRNAs had no noticeable effect on cell viability, even after induction periods of up to 10 days (data not shown).
FIG. 2.
Northern blot analysis of TbMT40 mRNA. The TbMT40 RNAi cell line was induced with TET for the indicated numbers of days, and poly(A)+ RNA was probed for TbMT40 mRNA (upper panel) and α-tubulin mRNA (tub; lower panel), which served as a loading control.
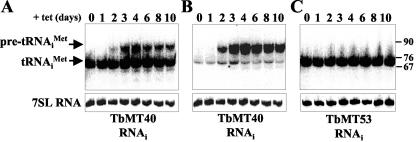
To test whether TbMT40 and/or TbMT53 was involved in the maturation of tRNAiMet, which would be similar to what has been described for yeast (2, 5), RNA samples were prepared 1, 2, 3, 4, 6, 8, and 10 days postinduction and subjected to Northern blotting (Fig. 3). A probe complementary to nt 47 to 64 of tRNAiMet detected two RNA species in TbMT40-silenced cells: the mature tRNAiMet of 71 nt and a species with a lower electrophoretic mobility of approximately 85 to 88 nt, indicative of a precursor molecule (Fig. 3A). Whereas the level of the mature tRNAiMet remained relatively constant during the time frame of the experiment, the larger molecule accumulated over time. It was first detected 2 days after induction and reached a maximum level 4 days after silencing TbMT40. In contrast, only the mature tRNAiMet was detected in TbMT53-silenced cells, and there was no evidence of precursor molecules (Fig. 3C). Furthermore, simultaneous silencing of TbMT40 and TbMT53 revealed an RNA pattern similar to that observed in TbMT40-silenced cells (data not shown). Thus, it appeared that down-regulation of TbMT53 had no detectable effect on the accumulation of tRNAiMet molecules.
FIG. 3.
Northern blot analysis of the effect of TbMT40 and TbMT53 RNAis on tRNAiMettranscript levels. The cell lines were induced for the indicated numbers of days, and total RNA was separated on a 15% acrylamide-7 M urea gel and was then hybridized to an oligonucleotide probe specific for mature tRNAiMet (nt 47 to 64) (A and C) or an oligonucleotide that predominantly recognizes tRNAiMet transcripts with 5′ extensions (B). The RNAs in all three blots were subsequently hybridized to a probe specific for 7SL RNA (lower panels) to control for loading variabilities. DNA nucleotide size markers are indicated on the right.
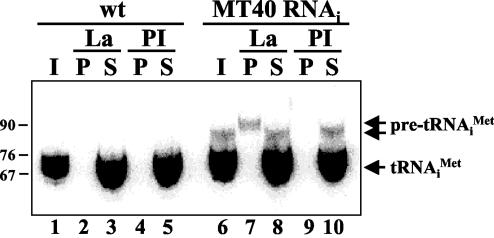
To elucidate the structure of the tRNAiMet precursor molecule addressed in Fig. 3A, RNA samples from TbMT40-silenced cells were hybridized with a probe specific for 5′ extensions. As can be seen in Fig. 3B, this probe predominantly detected the tRNAiMet precursor, demonstrating that the longer RNA molecule was not processed at the 5′ end. Next, we asked whether processing of the pre-tRNA transcript was also defective at the 3′ end in the absence of TbMT40. For this experiment, we prepared whole-cell extracts from control cells and TbMT40-silenced cells and subjected them to immunoprecipitations with antibodies directed against the T. brucei La protein (15). The rationale for this experiment was that La is known to bind to the 3′ trailer of pre-tRNAs, and thus precursors not processed at the 3′ end will be affinity selected with anti-La antibodies (28). Following immunoprecipitations, RNA was extracted from pellet and supernatant fractions and probed by Northern blotting with an oligonucleotide complementary to nt 47 to 64 of tRNAiMet (Fig. 4). As expected, the mature tRNAiMet was not affinity selected by the anti-La antibody. Similarly, in TbMT40-silenced cells, the tRNAiMet precursor with a 5′ extension was not detectable in the immunoprecipitate, suggesting that this molecule does not have a 3′ trailer. This conclusion is corroborated by the detection of an even longer precursor, which most likely has 5′ and 3′ extensions, in the La immunoprecipitate. This precursor was not evident in control cells and, thus, appeared to accumulate as a consequence of TbMT40 depletion, albeit to a much lower level than precursors with only 5′ extensions, since it is not detectable in the input RNAs. Taken together, our data showed that TbMT40 is required for the efficient processing of tRNAiMet and appeared to be involved mainly in the removal of 5′ extensions.
FIG. 4.
Immunoprecipitations with anti-La antibodies. Wild-type (wt) and induced TbMT40 RNAi cells were grown for 3 days, and total cell lysates were subjected to immunoprecipitations with preimmune sera (PI) and antibodies raised against the T. brucei La protein (La). RNA samples prepared from the total extracts (I) (lanes 1 and 6), the pellet fractions (P) (lanes 2, 4, 7, and 9), and the supernatant fractions (S) (lanes 3, 5, 8, and 10) were processed for Northern blot analysis with an oligonucleotide probe specific for mature tRNAiMet(nt 47 to 64). Note that only 10% of the total extract and supernatant was loaded. DNA nucleotide size markers are indicated on the left.
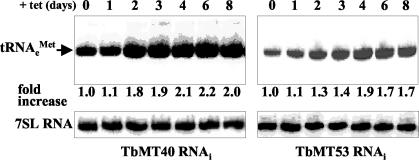
In yeast, it was shown that mutations in GCD10 or GCD14 had no effect on the processing or accumulation of elongator tRNAMet (2, 5). Similarly, we found that silencing of TbMT40 or TbMT53 did not lead to the accumulation of precursor molecules (Fig. 5). However, in contrast to the yeast results, we detected an approximately twofold increase in the amount of mature elongator tRNAMet over the 8-day period of induction. Interestingly, this effect was not only seen in TbMT40-silenced cells but was also apparent in TbMT53-silenced cells.
FIG. 5.
Northern blot analysis of the effect of TbMT40 and TbMT53 RNAi on elongator tRNAMet transcript levels. Cells were induced for the indicated numbers of days, and total RNA was hybridized to a probe complementary to nt 48 to 65 of elongator tRNAMet. Both blots were subsequently hybridized to a probe specific for 7SL RNA (lower panels) to control for loading variabilities. The levels of tRNA transcripts are presented as increases (n-fold) with respect to the amounts present at day 0.
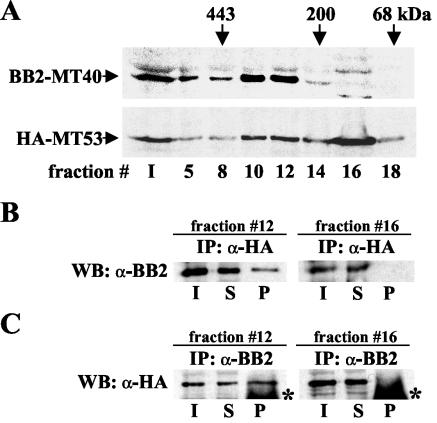
TbMT40 and TbMT53 are components of a nuclear 300-kDa complex.
In a separate set of experiments, one aimed at purifying enzymes involved in the synthesis of the trypanosomatid cap 4 structure, we noted that TbMT40 and TbMT53 cofractionated over several different chromatographic steps, suggesting that they were physically associated (data not shown). To corroborate this finding, we epitope tagged TbMT40 and TbMT53 in vivo with an N-terminal BB2 and HA tag, respectively, by homologous recombination with PCR-generated cassettes. In addition, the second allele of the TbMT40 gene was knocked out by inserting the blasticidin resistance gene. Thus, the resulting cell line expressed solely BB2-tagged MT40, whereas HA-tagged MT53 was expressed in the background of untagged MT53. Next, an S-100 extract was fractionated on a Superdex-200 gel filtration column, and selected fractions were assayed by Western blotting with anti-BB2 and anti-HA antibodies (Fig. 6A). The results showed that TbMT40 fractionated with an apparent molecular mass of approximately 300 kDa. The fractionation behavior of TbMT53 was more complex in that two peaks were apparent: one coincided with the TbMT40 peak (fractions 10 and 12), whereas a second peak was evident in fraction 16. We suspect that the latter peak is due to the fact that both HA-tagged and untagged MT53 are expressed in this cell line, and as a consequence a portion of the HA-tagged MT53 does not cofractionate with MT40. Nevertheless, to further substantiate a physical association of TbMT40 and TbMT53, fractions 12 and 16 of the Superdex-200 gel filtration column were subjected to immunoprecipitation with anti-HA or anti-BB2 antibodies (Fig. 6B and C). Western blotting of anti-HA immunoprecipitates (TbMT53) with anti-BB2 antibodies (TbMT40) showed that TbMT40 was coimmunoprecipitated with TbMT53 in fraction 12 but not in fraction 16 (Fig. 6B). Similarly, immunoprecipitation of TbMT40 with anti-BB2 antibodies resulted in the coselection of TbMT53 in fraction 12 but not in fraction 16 (Fig. 6C). Taken together, these data supported the notion that TbMT40 and TbMT53 are part of a complex with an apparent molecular mass of 300 kDa.
FIG. 6.
TbMT40 is in an approximately 300-kDa complex with TbMT53. (A) An S-100 extract from cells expressing BB2-tagged TbMT40 and HA-tagged TbMT53 was loaded onto a Superdex-200 column, and selected fractions were subjected to Western blot analysis with anti-BB2 or anti-HA antibodies. The elution positions of bovine serum albumin (68 kDa), β-amylase (200 kDa), and apoferritin (443 kDa) are indicated. (B) Fractions 12 and 16 from the results shown in panel A were immunoprecipitated with anti-HA antibodies (α-HA), and the input (I; 10% loaded), the supernatant (S; 10% loaded), and the precipitate (P; 100% loaded) were analyzed by Western blotting (WB) with anti-BB2 antibodies (α-BB2) to detect the presence of TbMT40. (C) As described for panel B, fractions 12 and 16 were immunoprecipitated with anti-BB2 antibodies and probed with anti-HA antibodies. The asterisks in lanes P indicate cross-reactivity with the immunoglobulin G heavy chain.
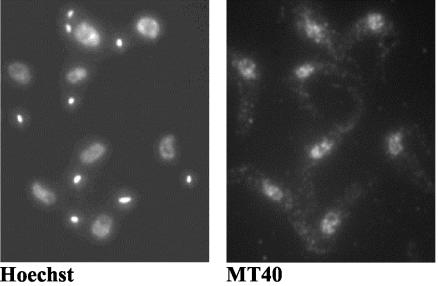
Finally, in order to determine the subcellular localization of TbMT40, anti-protein A antibodies were used to decorate T. brucei cells expressing TAP-tagged MT40 (see Materials and Methods). As shown in Fig. 7, TbMT40 was located predominantly in nuclei. The antibody staining was not homogeneously diffused throughout the nucleoplasm but was concentrated in a limited number of spherical bodies, which filled the entire nuclear interior, suggesting that the enzymes of the tRNA modification machinery are compartmentalized. Taken together, this series of experiments demonstrated that TbMT40 and TbMT53 are subunits of a protein complex that is localized predominantly in the nucleus.
FIG. 7.
Cellular localization of TbMT40. Procyclic cells expressing TAP-tagged TbMT40 were processed for indirect immunofluorescence by staining with a rabbit anti-protein A antibody (panel MT40). DNA was stained with Hoechst (panel Hoechst). The small dots represent the kinetoplast DNA.
DISCUSSION
This paper is the first report of a nuclear protein complex required for the maturation of tRNAiMet in Kinetoplastidae. Silencing by RNAi of one the components of this complex, namely, a 40-kDa polypeptide with signature motifs of SAM-dependent methyltransferases, resulted in the accumulation of two forms of precursor molecules. The dominant form retained extensions at the 5′ end but was processed at the 3′ end. In addition, a minor precursor was apparent in anti-La immunoprecipitates, which had both 5′ and 3′ extensions. These data point to a maturation pathway for T. brucei tRNAiMet, where 3′ trimming occurs before removal of the 5′ leader by RNase P. This is somewhat unusual for a eukaryotic organism, since in the majority of cases studied 3′ processing was seen only after 5′ processing. However, there are several reported exceptions to this rule, such as with silkworms (8) and mice (19). Furthermore, in the budding yeast, where numerous studies have led to the conclusion that 5′ maturation obligatorily precedes 3′ trimming, there was a recent report describing the reverse pathway for pre-tRNATrp (12). In the only other study of pre-tRNA maturation in parasitic protozoa, it was proposed that 5′ processing of T. brucei tRNATyr precedes 3′ processing (21). Thus, it remains to be seen whether the suggested pathway for pre-tRNAiMet is exceptional or represents the major processing order used in these organisms.
Silencing of TbMT40 by RNAi perturbed the maturation of tRNAiMet, which in some aspects mimics the effect of mutations in the S. cerevisiae GCD14 gene (2, 5). In both cases, there was an accumulation of precursors with extensions at both the 5′ and 3′ ends. However, whereas temperature-sensitive mutations in gcd14 lead to a reduction in mature tRNAiMet, this reduction was not observed in TbMT40-silenced cells. Most interestingly, silencing of TbMT40, as well as of TbMT53, the GCD10 homolog, resulted in a significant and reproducible accumulation of the mature elongator tRNAMet. This result highlights another difference with the yeast system, where mutations in GCD10 or GCD14 did not affect the levels of mature elongator tRNAMet (2, 5). At present, the significance of these subtle differences is not clear, and this will require further investigation. However, it should be pointed out that gene down-regulation by RNAi does not lead to a complete depletion of the proteins under study, whereas conditional gene knockout in yeast is a more powerful gene silencing system. Accordingly, RNAi of TbMT40 and TbMT53 or both does not detectably impair cell viability, whereas yeast GCD10 and GCD14 are essential genes. We have attempted and failed to generate a double TbMT40 gene knockout, suggesting that this gene is also essential in T. brucei (not shown).
With S. cerevisiae, it was demonstrated that the Gcd10p/Gcd14p complex is responsible for generating the m1A modification at position 58 of tRNAs (2, 3). This complex has been localized to the nucleus; however, based on the available evidence, we cannot exclude the possibility that Gcd10p and/or Gcd14p is also located in the cytoplasm (2). Our results are consistent with the yeast data in that TbMT40 was found predominantly in the nucleus. However, we also observed a punctate cytoplasmic staining, which may point to a function of TbMT40 in this cellular compartment. Experiments with S. cerevisiae revealed that the two subunits have distinct functions, with Gcd10p most likely being involved in binding the tRNA substrate and Gcd14p performing the modification. The latter point was established by introducing a mutation in motif I of the putative SAM-binding domain of Gcd14p, which abolished m1A methyltransferase activity (3). The three-dimensional structures of several methyltransferases revealed that motifs I and post-I directly contact SAM (18), and mutation of motif I impairs SAM binding (14). By analogy, we predict that the processing defects in the pre-tRNAiMet observed in TbMT40-silenced cells are due to the absence of m1A in tRNAs. At the sequence level, TbMT40 has the signature motifs of methyltransferases, namely, motifs I, post-I, II, and III (Fig. 1). In addition, TbMT40 is physically associated with TbMT53, a homolog of Gcd10p. The apparent molecular mass of approximately 300 kDa for the MT40/MT53 complex suggests that either additional, yet-to-be-identified proteins are part of this complex or that the 300-kDa complex is a multimeric structure, as it has been suggested for the yeast Gcd10/Gcd14 complex (3). Experiments are under way to address this issue and to characterize the function of this complex in the maturation of elongator tRNAMet.
Acknowledgments
This work was supported by NIH grant AI28798 to E.U. G.K.A. was supported through a training grant from the NIH (T32 AI07404).
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, J., L. Phan, R. Cuesta, B. A. Carlson, M. Pak, K. Asano, G. R. Bjork, M. Tamame, and A. G. Hinnebusch. 1998. The essential Gcd10p-Gcd14p nuclear complex is required for 1-methyladenosine modification and maturation of initiator methionyl-tRNA. Genes Dev. 12:3650-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, J., L. Phan, and A. G. Hinnebusch. 2000. The Gcd10p/Gcd14p complex is the essential two-subunit tRNA(1-methyladenosine) methyltransferase of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 97:5173-5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Astrom, S. U., and A. S. Bystrom. 1994. Rit1, a tRNA backbone-modifying enzyme that mediates initiator and elongator tRNA discrimination. Cell 79:535-546. [DOI] [PubMed] [Google Scholar]
- 5.Calvo, O., R. Cuesta, J. Anderson, N. Gutiérrez, M. T. García-Barrio, A. G. Hinnebusch, and M. Tamame. 1999. GCD14p, a repressor of GCN4 translation, cooperates with Gcd10p and Lhp1p in the maturation of initiator methionyl-tRNA in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:4167-4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Djikeng, A., H. Shi, C. Tschudi, S. Shen, and E. Ullu. 2003. An siRNA ribonucleoprotein is found associated with polyribosomes in Trypanosoma brucei. RNA 9:802-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank, D. N., and N. R. Pace. 1998. Ribonuclease P: unity and diversity in a tRNA processing ribozyme. Annu. Rev. Biochem. 67:153-180. [DOI] [PubMed] [Google Scholar]
- 8.Garber, R. L., and L. P. Gage. 1979. Transcription of a cloned Bombyx mori tRNA2Ala gene: nucleotide sequence of the tRNA precursor and its processing in vitro. Cell 18:817-828. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Barrio, M. T., T. Naranda, C. R. Vazquez de Aldana, R. Cuesta, A. G. Hinnebusch, J. W. Hershey, and M. Tamame. 1995. GCD10, a translational repressor of GCN4, is the RNA-binding subunit of eukaryotic translation initiation factor-3. Genes Dev. 9:1781-1796. [DOI] [PubMed] [Google Scholar]
- 10.Hinnebusch, A. G. 1997. Translational regulation of yeast GCN4. A window on factors that control initiator-tRNA binding to the ribosome. J. Biol. Chem. 272:21661-21664. [DOI] [PubMed] [Google Scholar]
- 11.Kagan, R. M., and S. Clarke. 1994. Widespread occurrence of three sequence motifs in diverse S-adenosylmethionine-dependent methyltransferases suggests a common structure for these enzymes. Arch. Biochem. Biophys. 310:417-427. [DOI] [PubMed] [Google Scholar]
- 12.Kufel, J., and D. Tollervey. 2003. 3′-processing of yeast tRNATrp precedes 5′-processing. RNA 9:202-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeBlanc, A. J., A. E. Yermovsky-Kammerer, and S. L. Hajduk. 1999. A nuclear encoded and mitochondrial imported dicistronic tRNA precursor in Trypanosoma brucei. J. Biol. Chem. 274:21071-21077. [DOI] [PubMed] [Google Scholar]
- 14.Mao, X., B. Schwer, and S. Shuman. 1996. Mutational analysis of the Saccharomyces cerevisiae ABD1 gene: cap methyltransferase activity is essential for cell growth. Mol. Cell. Biol. 16:475-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchetti, M. A., C. Tschudi, H. Kwon, S. L. Wolin, and E. Ullu. 2000. Import of proteins into the trypanosome nucleus and their distribution at karyokinesis. J. Cell Sci. 113:899-906. [DOI] [PubMed] [Google Scholar]
- 16.Morl, M., and A. Marchfelder. 2001. The final cut. The importance of tRNA 3′-processing. EMBO Rep. 2:17-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ngo, H., C. Tschudi, K. Gull, and E. Ullu. 1998. Double-stranded RNA induces mRNA degradation in Trypanosoma brucei. Proc. Natl. Acad. Sci. USA 95:14687-14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niewmierzycka, A., and S. Clarke. 1999. S-adenosylmethionine-dependent methylation in Saccharomyces cerevisiae. Identification of a novel protein arginine methyltransferase. J. Biol. Chem. 274:814-824. [DOI] [PubMed] [Google Scholar]
- 19.Rooney, R. J., and J. D. Harding. 1986. Processing of mammalian tRNA transcripts in vitro: different pre-tRNAs are processed along alternative pathways that contain a common rate-limiting step. Nucleic Acids Res. 14:4849-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider, A., and L. Marechal-Drouard. 2000. Mitochondrial tRNA import: are there distinct mechanisms? Trends Cell Biol. 10:509-513. [DOI] [PubMed] [Google Scholar]
- 21.Schneider, A., K. P. McNally, and N. Agabian. 1993. Splicing and 3′-processing of the tyrosine tRNA of Trypanosoma brucei. J. Biol. Chem. 268:21868-21874. [PubMed] [Google Scholar]
- 22.Shen, S., G. K. Arhin, E. Ullu, and C. Tschudi. 2001. In vivo epitope tagging of Trypanosoma brucei genes using a one step PCR-based strategy. Mol. Biochem. Parasitol. 113:171-173. [DOI] [PubMed] [Google Scholar]
- 23.Shi, H., A. Djikeng, C. Tschudi, and E. Ullu. 2004. Argonaute protein in the early divergent eukaryote Trypanosoma brucei: control of small interfering RNA accumulation and retroposon transcript abundance. Mol. Cell. Biol. 24:420-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sprinzl, M., C. Horn, M. Brown, A. Ioudovitch, and S. Steinberg. 1998. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 26:148-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan, T. H., N. Bochud-Allemann, E. K. Horn, and A. Schneider. 2002. Eukaryotic-type elongator tRNAMet of Trypanosoma brucei becomes formylated after import into mitochondria. Proc. Natl. Acad. Sci. USA 99:1152-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tschudi, C., A. Djikeng, H. Shi, and E. Ullu. 2003. In vivo analysis of the RNA interference mechanism in Trypanosoma brucei. Methods 30:304-312. [DOI] [PubMed] [Google Scholar]
- 27.Wirtz, E., S. Leal, C. Ochatt, and G. A. Cross. 1999. A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol. Biochem. Parasitol. 99:89-101. [DOI] [PubMed] [Google Scholar]
- 28.Wolin, S. L., and T. Cedervall. 2002. The La protein. Annu. Rev. Biochem. 71:375-403. [DOI] [PubMed] [Google Scholar]
- 29.Yoo, C. J., and S. L. Wolin. 1997. The yeast La protein is required for the 3′ endonucleolytic cleavage that matures tRNA precursors. Cell 89:393-402. [DOI] [PubMed] [Google Scholar]