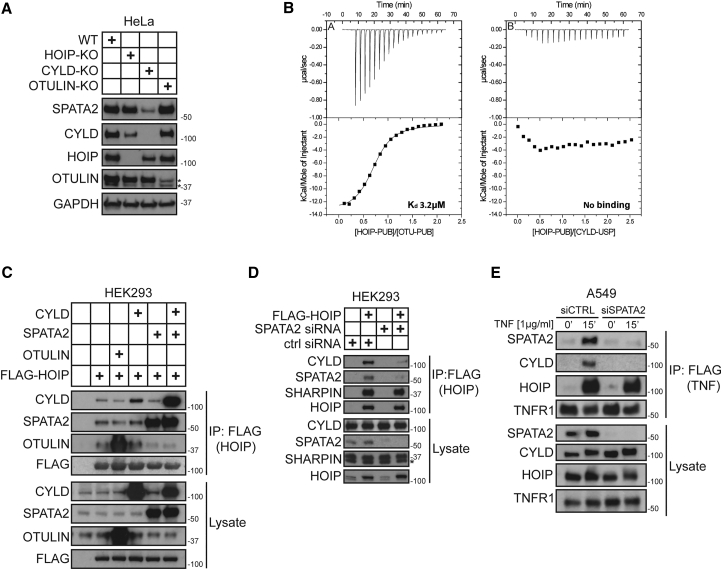
Figure 3.
Binding of CYLD to HOIP and Its Recruitment to the TNFR1-SC Requires SPATA2
(A) HOIP, CYLD, or OTULIN were knocked out via clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 and levels of the indicated proteins compared to HeLa WT by western blot analysis.
(B) ITC characterization of the interactions between (A) the HOIP-PUB domain (597 μM) and a peptide derived from the PIM motif (58 μM) of OTULIN and (B) the HOIP-PUB domain (398 μM) and the CYLD-USP domain (40 μM). For each titration, the raw data and normalized integrated heats are reported.
(C) HEK293 cells were transfected with different combinations of CYLD, SPATA2, and OTULIN together with FLAG-HOIP. Protein complexes were subsequently purified using anti-FLAG beads and analyzed by western blotting.
(D) SPATA2 expression was suppressed in FLAG-HOIP transfected HEK293 cells using siRNA. FLAG-HOIP was subsequently immunoprecipitated and tested for associated proteins by western blot analysis.
(E) A549 cells were transfected with control or SPATA2 siRNA. After 72 hr, cells were subjected to TNFR1-SC purification and analyzed by western blotting.