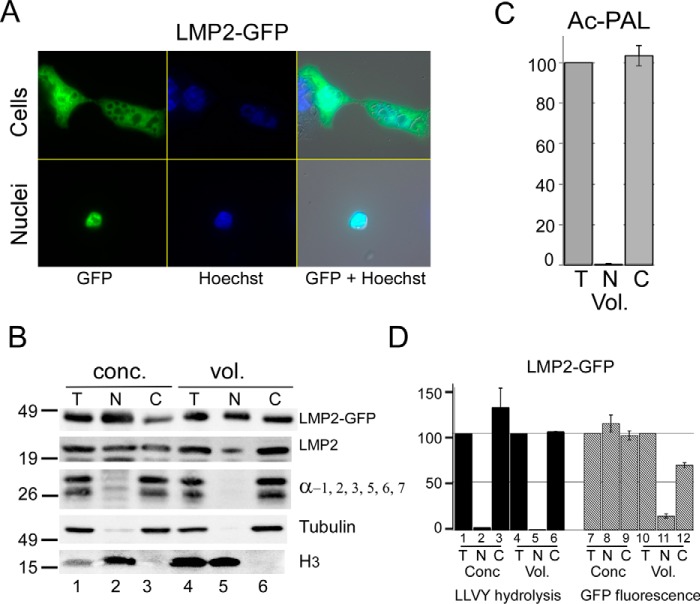
FIGURE 6.
Catalytic activity of the immunoproteasome is restricted to the cytosol. A, LMP2-GFP was expressed in HEK293T cells, and distribution was observed throughout the cells (upper panels). Purified nuclei also showed LMP2-GFP fluorescence (lower panels). B, fractionated extracts were examined by SDS-PAGE and immunoblotting. LMP2-GFP and native LMP2 were both detected in nuclear (N) and cytosolic (C) fractions, whereas subunits of the 26S proteasome showed strong cytosolic localization (α-1, 2, 3, 5, 6, 7). Histone H3 was detected only in the nuclear fraction, and tubulin was found in the cytosol. Loading equal concentration (conc.) of protein over-samples nuclear proteins, as noted by the higher level of histone H3 in the nucleus (N, lane 2) compared with total lysate (T, lane 1). In contrast, when proportional volumes (vol.) are examined, nuclear (N) and total (T) levels of H3 are similar. C, hydrolysis of Ac-PAL-AMC, which measures immunoproteasome peptidase activity, was only detected in the cytosolic fraction. D, in agreement with earlier results, LLVY-AMC hydrolysis occurred exclusively in the cytosolic fraction. The level of LMP2-GFP fluorescence was determined in the same fractionated extracts, and localization of LMP2-GFP in the nuclear fraction was verified. (Quantitation reflects S.E. from three independent experiments.)