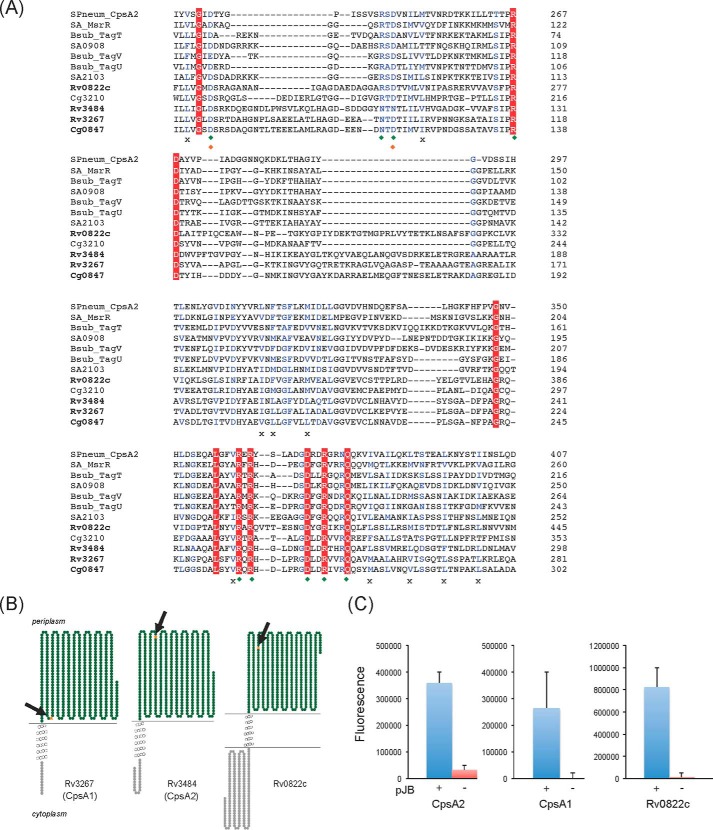
FIGURE 2.
Sequence and transmembrane topology of M. tuberculosis LCP protein candidates. A, alignment of the LytR-CpsA-Psr domains of LCP proteins and LCP protein candidates from M. tuberculosis, C. glutamicum, S. aureus, B. subtilis, and Streptococcus pneumoniae using Clustal Omega. Amino acids that are invariant in the alignment are colored white with a red background; homologous residues are in blue. Green diamonds, charged residues that contact the pyrophosphate headgroup of bound octaprenyl pyrophosphate in the crystal structure of CpsA2 from S. pneumoniae; orange diamonds, residues that coordinate a magnesium ion; crosses, conserved hydrophobic residues involved in the binding of the polyisoprenoid chain. B, transmembrane topology of Rv3267 (CpsA1), Rv3484 (CpsA2) and Rv0822c. The models were generated using TOPO2. C, topology of the C-terminal LCP domains of CpsA1, CpsA2, and Rv0822c in M. smegmatis. The LCP domains of the cpsA1, cpsA2, and Rv0822c genes were fused in frame with gfp in pJB(−) (red bars) and pJB(+) (blue bars). The positions of the GFP fusions in each protein are indicated by arrows and orange stars in the models shown in B. Fluorescence intensities were normalized to the A600 of the cultures, and the results shown represent the means and S.D. values of fluorescence intensities determined on at least 3–5 independent M. smegmatis transformants for each pJB(−) and pJB(+) plasmid. The addition of a single transmembrane domain from glycophorin A between the C-terminal fusion point of the protein of interest and the GFP in pJB(+) allows membrane-associated proteins with extracellular C-terminal fusions to be converted to proteins with intracellular C-terminal fusions. The native topology is reported with the fusion junction lacking the glycophorin A single transmembrane domain in the pJB(−) plasmid. Because GFP fluoresces in the cytoplasm but not in the periplasm, a high fluorescence signal in the pJB(+) version and background fluorescence in the pJB(−) version, as is the case with all three proteins here, are indicative of the C-terminal fusion of the protein being periplasmic. Error bars, S.D.