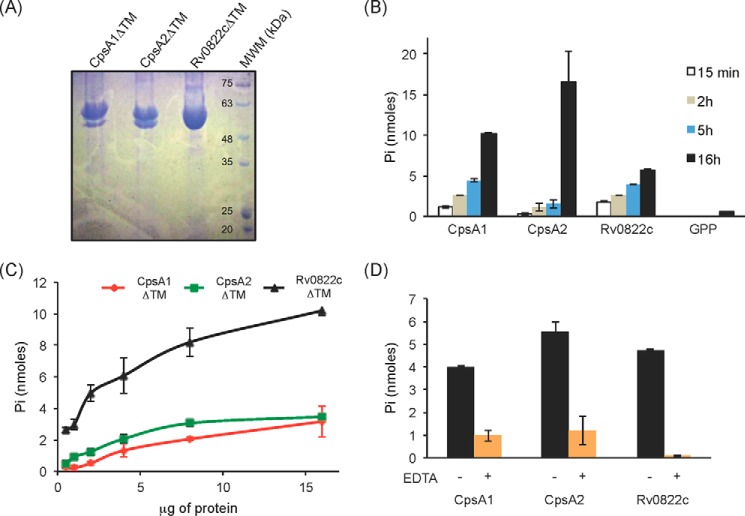
FIGURE 3.
Pyrophosphatase activity of the three LCP proteins of M. tuberculosis recombinantly expressed and purified from E. coli. A, Coomassie Blue-stained SDS-polyacrylamide gel showing the purified CpsA1, CpsA2, and Rv0822c proteins devoid of transmembrane domain (CpsA1ΔTM, CpsA2ΔTM, and Rv0822cΔTM) upon affinity chromatography. The expected size of CpsA1ΔTM is 47.7 kDa, that of CpsA2ΔTM is 51.5 kDa, and that of Rv0822cΔTM is 52.4 kDa. Some degradation was consistently seen with CpsA1ΔTM and CpsA2ΔTM. The bottom bands have been confirmed to be proteolytic truncations of the target enzyme. B–D, pyrophosphatase assays. This assay monitors spectrophotometrically the hydrolysis of the pyrophosphate phosphoanhydride bond of GPP releasing Pi. B, the activity of the three purified LCP proteins is time-dependent. Reaction mixtures contained 5 μg (Rv0822cΔTM) or 10 μg (CpsA1ΔTM and CpsA2ΔTM) of recombinant proteins and were incubated for 15 min, 2 h, 5 h, and 16 h. C, protein concentration dependence. Reaction mixtures contained 0.5–16 μg of recombinant proteins and were incubated for 16 h. D, reaction mixtures contained 5 μg of recombinant protein and were incubated for 16 h in the absence (black bars) or presence (orange bars) of 10 mm EDTA. All assays were performed at least twice on independent recombinant protein preparations. Shown are the averages and S.D. values (error bars) of enzyme activities measured in duplicate in one representative experiment.