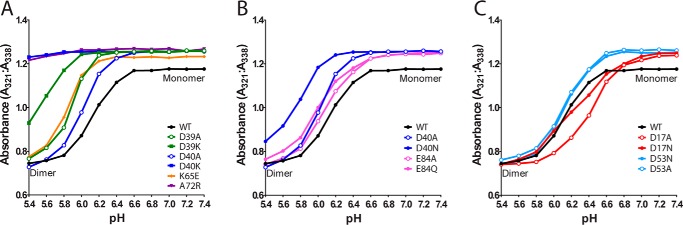
FIGURE 6.
Structure-function analysis of residues involved in NcNTD dimerization. During transition of the NTD from monomer to dimer, the single tryptophan residue of the NTD (Trp10) undergoes a conformational change that increases its solvent exposure and results in quenching of its intrinsic fluorescence emission. The ratio of the tryptophan fluorescence signals at 321–338 nm reflects the ratio between monomeric (338 nm) and dimeric (321 nm) NcNTD. A–C, the tryptophan fluorescence ratio of the signals at 321 and 338 nm was plotted as a function of pH for WT, A72R, and mutants intended to affect the Asp39-Lys65 and Asp40-Lys65 salt bridges (A), mutants probing the Asp40-Glu84 handshake interaction (B), and mutants probing the Asp17-Asp53 handshake interaction (C). Experiments were performed in triplicate, and representative raw data are shown in supplemental Fig. S1.