FIGURE 5.
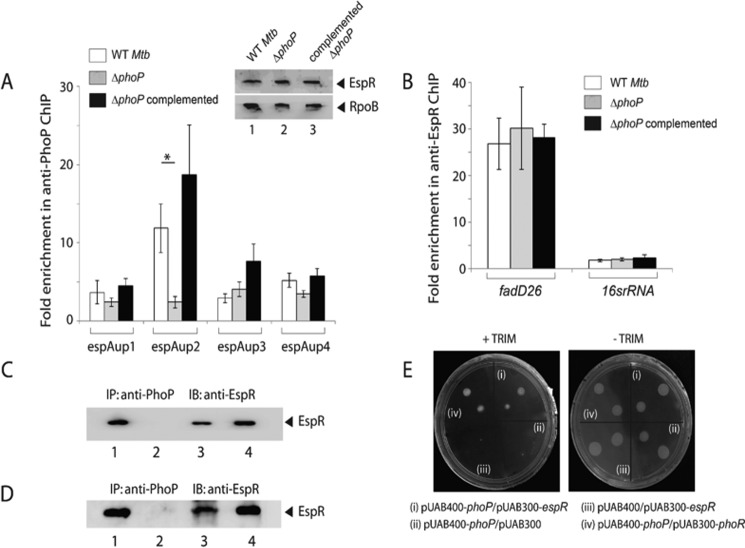
Recruitment of EspR at the espACD promoter requires the presence of PhoP. A, ChIP-qPCR was carried out to assess EspR recruitment to the espACD promoter of WT, ΔphoP, and the complemented mutant. Fold PCR enrichment due to EspR binding was determined against PCR signal from the mock sample generated by an IP experiment without adding antibody. Note that anti-EspR ChIP data of Fig. 3C was included here to enable a direct comparison of results from WT and ΔphoP M. tuberculosis. Each data for ChIP experiments represent the mean of duplicate qPCR measurements using at least three independent M. tuberculosis cultures. Asterisk indicates a statistically significant difference in the ChIP enrichment at espAup2 compared between WT M. tuberculosis and the phoP mutant (*, p < 0.05). Inset shows comparable EspR expression in WT, ΔphoP, and complemented ΔphoP as determined by immunoblotting of crude cell lysates (∼20 μg of total protein) using anti-EspR antibody; RpoB was used as a loading control. B, to examine specificity of in vivo EspR recruitment, ChIP-qPCR experiments utilized fadD26 promoter as a positive control using appropriate primer pair (supplemental Table S2). Specificity of enrichment was also verified with 16S rRNA gene-specific primer (supplemental Table S2) using identical anti-EspR IP samples from the WT and the mutant M. tuberculosis strains. C, to examine PhoP-EspR interaction in vivo, crude cell lysates of WT M. tuberculosis H37Rv were immunoprecipitated with anti-PhoP antibody, and IP samples were visualized by immunoblotting with anti-EspR antibody; lane 1, input sample; lane 2, control with mock IP (without adding antibody), lane 3, anti-PhoP IP of crude lysate, and lane 4, recombinant EspR as a positive control. D, to further examine PhoP-EspR interaction in vitro, crude cell lysates of E. coli BL21(DE3) expressing both PhoP and EspR via pRSF-Duet-1 vector as described under the “Experimental Procedures” was immunoprecipitated with anti-PhoP antibody, and IP samples were visualized by Western blot analysis using anti-EspR antibody. The lane composition of the SDS-PAGE remains identical to that of C. E, M-PFC experiment to study interaction of PhoP and EspR involved co-expression of indicated fusion constructs in M. smegmatis, used as a surrogate host. Growth of transformants on 7H10/kanamycin/hygromicin hygromycin in presence of TRIM confirms in vivo protein-protein association between PhoP and EspR. Co-expression of empty vectors and phoP/phoR pair were included as negative and positive control, respectively. All of these strains grew well in absence of TRIM. IB, immunoblot.