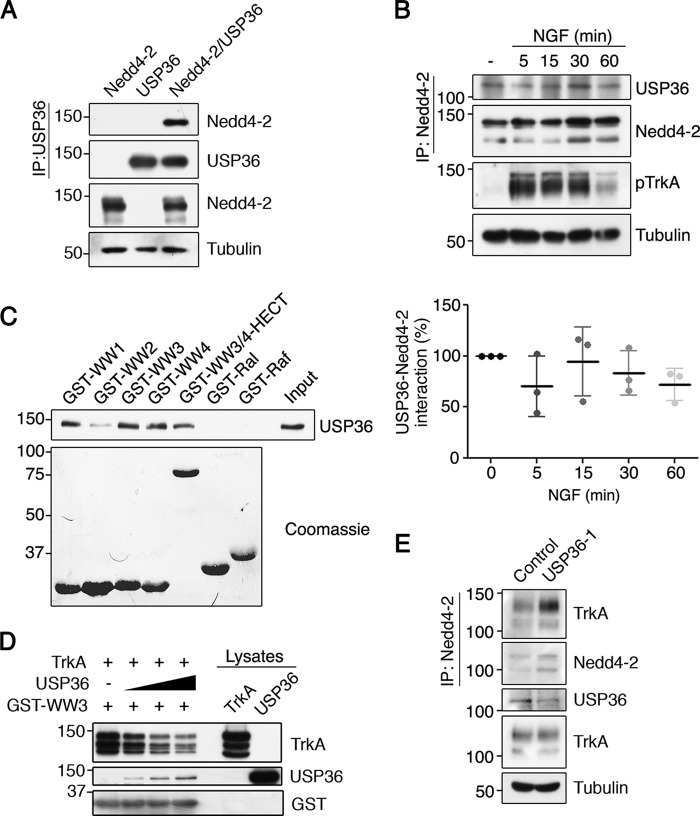
FIGURE 5.
USP36 interacts with Nedd4-2 through WW domains and competes TrkA binding to Nedd4-2. A, USP36 and Nedd4-2 interact. Lysates from HEK293 cells transfected with GFP-Nedd4-2, FLAG-USP36, or both plasmids together were subjected to immunoprecipitation (IP) using FLAG antibodies. Western blotting was performed to assess Nedd4-2 co-immunoprecipitation and USP36. The expression levels of Nedd4-2 and tubulin as a loading control are shown. A representative experiment is shown (n = 3). B, Nedd4-2 and USP36 interact independently of NGF. Cell lysates from PC12-6/15 cells stimulated or not with NGF for different time points were subjected to immunoprecipitation with Nedd4-2 antibodies and subjected to Western blotting analysis to detect the presence of USP36 in the immunoprecipitate. Active TrkA (pTrkA) and tubulin in cell lysates were probed to assess NGF treatment and loading, respectively. A representative experiment is shown (top panels). Quantification of USP36-Nedd4-2 interaction is shown (bottom panel) (means ± S.D.; n = 3). C, WW domains of Nedd4-2 mediate the interaction with USP36. Recombinant proteins containing the GST-WW domains (GST-WW1, GST-WW2, GST-WW3, and GST-WW4), WW3,4-HECT, and GST-Ral and GST-Raf binding domains were incubated with lysates containing USP36 and subjected to Western blotting analysis with FLAG antibodies (top panel). A Coomassie-stained gel is shown to detect GST fusion proteins (bottom panel). A representative experiment is shown (n = 2). Note the association of USP36 with WW1, 3, and 4 and WW3/4-HECT. D, USP36 competes with TrkA binding to Nedd4-2. A recombinant protein GST-WW3 domain was incubated with a fixed amount of TrkA and increasing amounts of USP36 and subjected to Western blotting analysis with different antibodies. A representative experiment is shown (n = 3). Note the displacement of TrkA binding to GST-WW3 and the increased binding of USP36 when increasing amounts of USP36 were added. E, TrkA and Nedd4-2 interaction is modulated by USP36 levels. Lysates from PC12-6/15 cells infected with lentivirus expressing control shRNA or USP36 shRNA-1 were subjected to immunoprecipitation using Nedd4-2 antibodies. Western blotting was performed to assess TrkA co-immunoprecipitation and Nedd4-2. The expression levels of TrkA, USP36, and tubulin as a control loading are shown. A representative experiment is shown (n = 3). Note the increased amount of TrkA co-immunoprecipitated with Nedd4-2 when USP36 levels are reduced.