FIGURE 1.
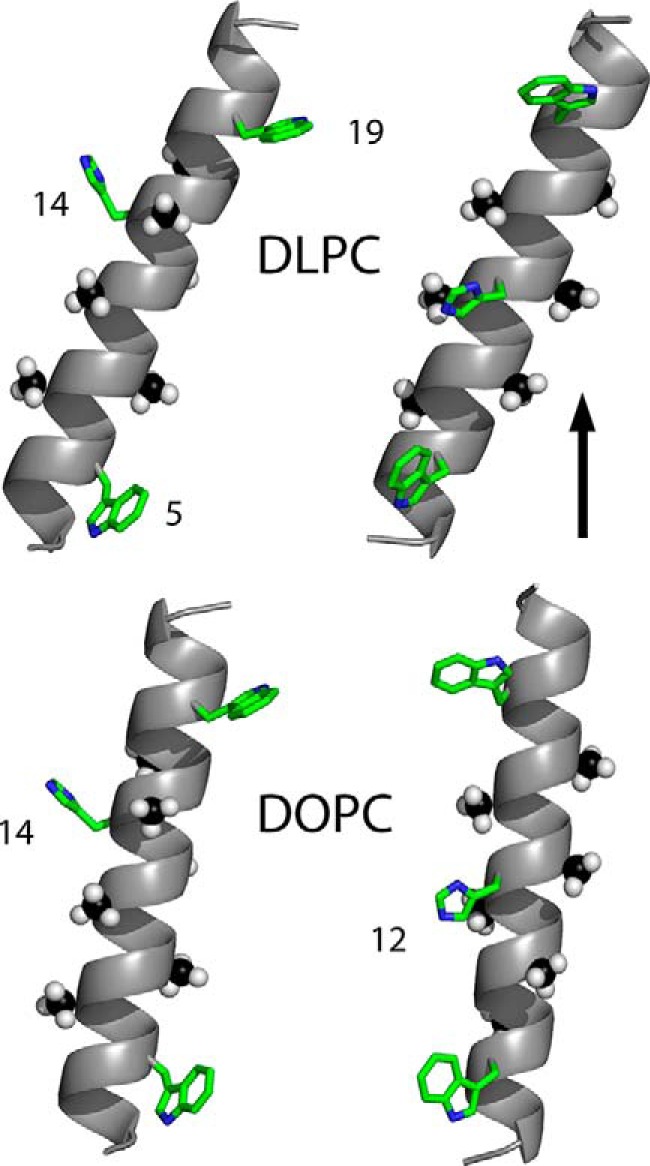
Models to illustrate the experimental tilted orientations of charged GWALP23-H14 (left) or neutral GWALP23-H12 (right), with respect to a vertical bilayer normal (see arrow) of DLPC or DOPC lipid bilayer membranes. The peptide with the charged His14 side chain is more tilted than is GWALP23-H12 in both membranes. In addition to rotational differences, as illustrated, each peptide helix is more tilted in the thinner DLPC bilayer (upper models) than in the thicker DOPC bilayer. The numbers indicate tryptophans 5 and 19, and histidines 12 and 14, in the peptide sequences. The six deuterated alanine methyl groups that underlie the tilt analysis are shown as space filling. Carbons are either green or black, nitrogens are blue, and hydrogens are white. The experimental helix tilt magnitudes and the pKa values of the histidine side chains are explained under “Results.”
