FIGURE 8.
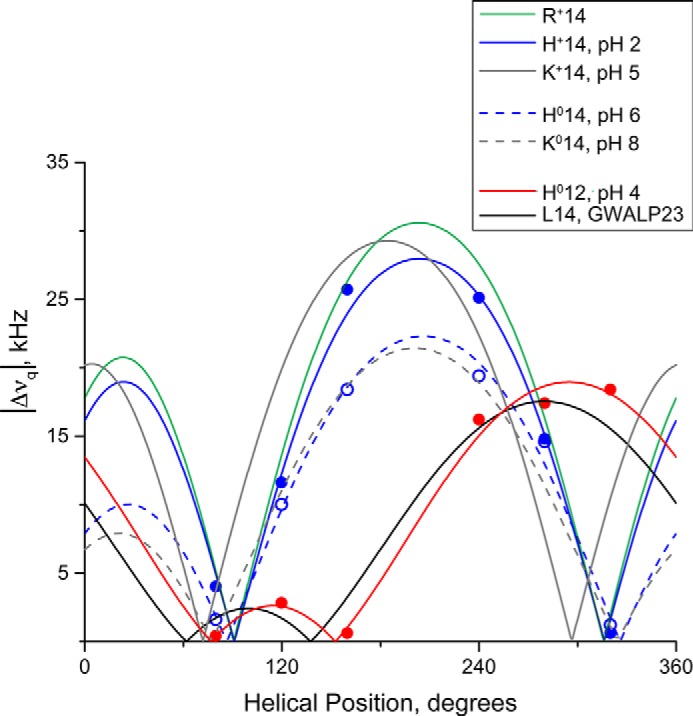
Quadrupolar wave analysis of tilted transmembrane peptides in DOPC bilayers. GWALP23-H14 (blue dashed line; tilt τ = 10°, rotation ρ = 250°, pH 5.9) is similar to neutral Y5GWALP23-K14 (gray dashed line; tilt τ = 9°, rotation ρ = 244°, pH 8.2) (24) indicating that the His14 residue is uncharged. At pH 2.0, the His residue of GWALP23-H14 (blue; tilt τ = 15°, rotation ρ = 247°) has a similar orientation to charged GWALP23-R14 (green; tilt τ = 15°, rotation ρ = 247°) (25) and charged Y5GWALP23-K14 (gray; tilt τ = 15°, rotation ρ = 228°, pH 5.2) (24), indicating that the His residue is charged. Note the similar rotation for all peptides with either Arg, His, or Lys at position 14, regardless of charge, indicating a change in rotation of ∼70° when compared with GWALP23 (black; tilt τ = 6°, rotation ρ = 323°) in response to the mutation of Leu14 to a polar residue. Changing the charge state of residue 14 is reflected by a change in peptide tilt in DOPC.
