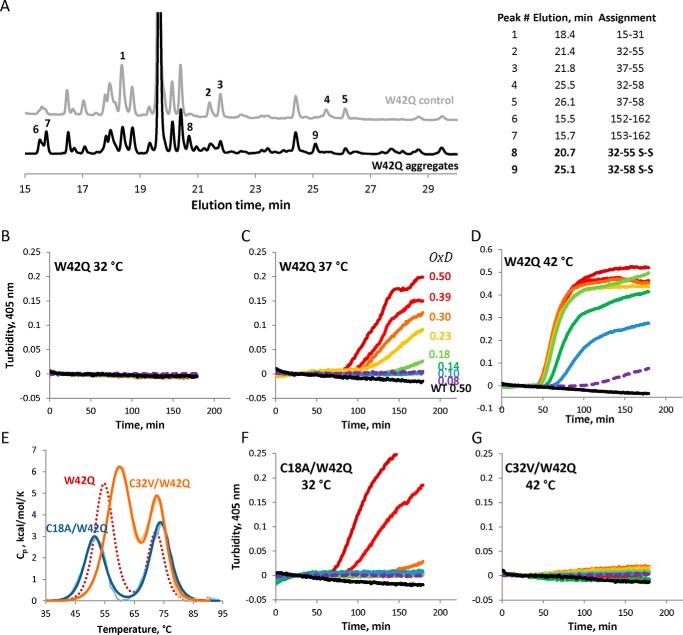
FIGURE 7.
Cys32–Cys41 is the predominant internal disulfide enabling W42Q aggregation. A, HPLC traces of aggregated (OxD = 0.2) and control (non-oxidized) W42Q samples following trypsin digestion under non-reducing conditions. Assignments for selected peaks, using electrospray mass spectrometry, are shown at the right. B–D, oxidative aggregation of W42Q is highly temperature-dependent in assays carried out as in Fig. 1. E, Cys mutations in the W42Q N-terminal domain alter its thermostability. Fitting for the W42Q DSC unfolding shown in red (dashed line) from Fig. 1C is shown for comparison. F and G, temperatures for the OxD aggregation assays on the C18A/W42Q (F) and C32V/W42Q (G) constructs were chosen to control for the differential thermostability. The former mutant aggregates already at 32 °C, whereas the latter does not do so even at 42 °C.