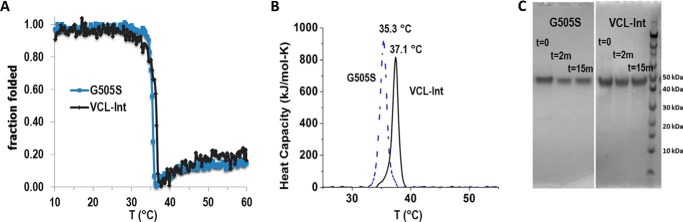
FIGURE 2.
Structural characterization of VCL-Int and a representative mutated construct (G505S). A, CD thermal transitions of VCL-Int and G505S showing triple helix unfolding around 37 °C. The increase in ellipticity after 37 °C is due to the unfolding of the α-helix in the V domain. B, differential scanning calorimetry analysis showing thermal transitions of 37.1 °C for VCL-Int and 35.3 °C for G505S. C, SDS-PAGE of VCL-Int and VCL-Int G505S after trypsin digestion for time t = 0, 2, and 15 min at 20 °C. Similar results were observed for G496S, G499S, G502S, G508S, and G511S (data not shown). A small initial drop in intensity is observed that could reflect rapid digestion of a small amount of impurity or unfolded collagen. There is little change in the intensity following this initial decrease, suggesting that the mutations did not lead to major unfolding.