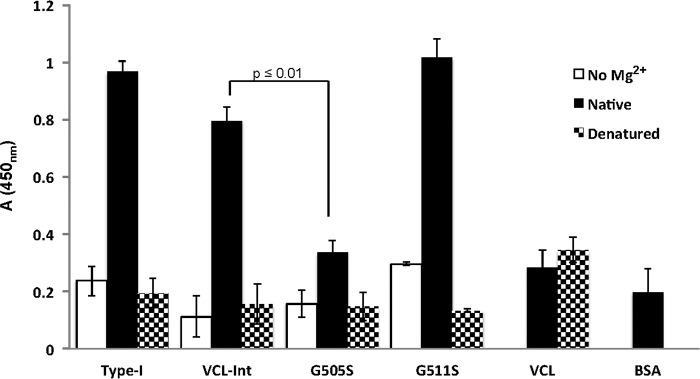
FIGURE 3.
Solid-state analysis of recombinant integrin α2 I domain binding to native and denatured recombinant collagens that are immobilized on 96-well plates and incubated with recombinant I domain (10 μg/ml). Anti-GST antibody was used to detect the bound I domain. Proteins were immobilized in their native state (black columns), in the denatured state (dotted columns), and in the absence of the Mg2+ cation (white columns). The native VCL showed no binding, whereas native VCL-Int showed a high binding similar to native type I collagen. Native G505S had a low binding, whereas native G511S had a binding similar to VCL-Int. All proteins in their denatured states showed no binding as triple helicity is essential for integrin binding. The divalent cation (Mg2+) is essential for the MIDAS motif, and BSA was used as a background value. Statistical analysis was performed by paired t test. The binding of G505S was significantly different from the binding of VCL-Int (p ≤ 0.01). All experiments were in triplicate, and error bars represent the standard deviation.