Abstract
Background
Granulomatosis with polyangiitis (GPA) is associated with an increased risk of mortality; however, recent mortality trends in GPA are unknown. We evaluated this issue in a general population context.
Methods
Using data collected between 1992 and 2013 by The Health Improvement Network in the United Kingdom, we identified individuals diagnosed as incident cases of GPA and up to 10 non-GPA controls matched on sex, age, year of birth, and year of GPA diagnosis. The cohort was divided into two based on the year of diagnosis (i.e., 1992–2002 and 2003–2013) to evaluate changes in mortality. We calculated hazard ratios for death using a Cox-proportional hazards model and the rate differences using an additive hazard model, while adjusting for potential confounders.
Results
We identified 465 cases of GPA (mean age, 60 years; 52% male). The early cohort (1992–2002) GPA patients had considerably higher mortality rates than the late cohort (2003–2013) (i.e., 72.0 versus 35.7 cases per 1000 person-years), as compared with a moderate improvement in the comparison cohorts between the two periods (19.8 versus 17.0 cases per 1000 person-years). The corresponding absolute mortality rate difference was 52.2 (95% CI, 25.1–79.2) cases and 18.7 (95% CI, 8.3–29.1) cases per 1000 person-years (p for interaction=0.025). The resulting HRs for mortality were 4.34 (95% CI, 2.72–6.92) and 2.41 (95% CI, 1.74–3.34), respectively (p for interaction=0.043).
Conclusion
This population-based study suggests that survival of GPA patients has improved considerably over the past two decades, affirming the benefits of recent trends in the management of GPA and its complications.
Keywords: Autoantibody, Granulomatosis with polyangiitis, epidemiology, cyclophosphamide
INTRODUCTION
Granulomatosis with polyangiitis (GPA; formerly Wegener’s Granulomatosis (1)) is a type of anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) typically associated with necrotizing, granulomatous inflammation. It has a predilection for the upper and lower airways as well as the kidneys, where it is a cause of rapidly progressive glomerulonephritis. Prior reports describe higher mortality in patients with GPA (and other forms of AAV) compared to the general population, standardized mortality rate (SMR) estimates ranging from 2.1 to 4.8 (2–9).
The addition of cyclophosphamide to glucocorticoids in the treatment of GPA transformed the prognosis from a uniformly fatal disease to a chronic condition (10, 11), complicated by treatment-related morbidity such as infections and malignancies (4, 11, 12, 13). Over the last two decades, management strategies have tended to minimize cumulative doses of cyclophosphamide (14) in favor of medications such as azathioprine and methotrexate (15–18). The goals of the use of these medications have been two-fold: to decrease the duration of patients’ exposure to cyclophosphamide and to minimize the quantity of glucocorticoids required to maintain disease remission. Coincident with these changes, Watts et al. reported an increasing prevalence of GPA that is likely multifactorial, related in part to greater awareness of the disease and improved diagnostic approaches, but also perhaps to improved survival (19).
To date, no study has directly evaluated whether mortality trends have improved over the past several decades, although the management of GPA and its complications has evolved substantially. We sought to address this key knowledge gap in a general population context.
METHODS
Data Source
Data were obtained from The Health Improvement Network (THIN) which includes data on approximately 7.3 million patients derived from 477 general practices in the United Kingdom (UK) (20). Because the National Health Service (NHS) in the UK requires every individual to be registered with a general practitioner (GP) regardless of health status, THIN is a population-based cohort representative of the UK general population. During clinical visits, data on diagnoses, prescription medications, height, weight, smoking status, and other variables are entered by providers. The Read classification is used to code specific diagnoses (21) and a drug dictionary based on data from the Multilex classification is used to code drugs (http://www.fdbhealth.com/multiplex-overview/1/26/2015). This study was approved by the THIN Scientific Review Committee.
Study Design
We performed a matched cohort study to examine the relationship of patients with incident GPA with the risk of all-cause mortality. For each GPA case, we selected up to 10 individuals (without GPA at the index date of GPA onset) and matched them to cases by sex, age, year of birth, and data-entry date (same month and year). The eligible population included all patients aged 20 to 90 years who had been enrolled in the THIN database between 1992 and 2013 for at least one continuous year prior to the index date; those with a previous diagnosis of GPA were excluded. We then divided the cohort into two calendar-time based sub-cohorts, based on the year of diagnosis (i.e., 1992–2002 [early cohort] and 2003–2013 [recent cohort]) to evaluate changes in mortality. These time periods of 11 years were chosen based on the timing of publications between 2000 to 2003 studying the safety and efficacy of reduced cyclophosphamide exposure and steroid-sparing agents (e.g., azathioprine, methotrexate) for maintenance therapy (14, 17). We followed patients included in this study from the date of diagnosis and reference participants from the matched (index) date until occurrence of death, migration out of the THIN database, or December 31, 2013, whichever came first.
Ascertainment of GPA
GPA was defined using Read codes for Wegener’s granulomatosis and Wegener’s syndrome, a strategy that has been previously validated (19). Cases were defined as those with a first diagnosis of GPA entered into the record during the period from January 1, 1992 through December 31, 2013. The entry date was considered to be the date at which a first diagnosis of GPA was given. A cushion period of a minimum of 1 year was used to identify incident cases of GPA.
Assessment of Covariates
From the THIN database, we collected data on personal characteristics and lifestyle factors such as smoking status, body mass index (BMI), alcohol use, healthcare utilization (i.e., GP visits), social-economic deprivation index (SDI) and comorbidities (i.e., chronic kidney disease, hypertension, stroke, hyperlipidemia, and diabetes). The Charlson Comorbidity Index (CCI) (22) prior to the index date was calculated. BMI, smoking status, and alcohol consumption were recorded to the nearest possible measurement prior to the index date. Similarly, we assessed baseline use of the following medications commonly prescribed to treat cardiovascular disease which have affected mortality rates: angiotensin converting enzyme inhibitors, beta-blockers, aspirin, angiotensin II receptor blockers, and calcium channel blockers. We also assessed baseline use of diuretics, non-steroidal anti-inflammatory drugs, and glucocorticoids.
Statistical Analysis
We compared the baseline characteristics of patients with GPA and the comparison cohort. Person-years of follow up for each patient were computed at the time from the index date to the end of follow-up. We calculated incidence rates of death for each group by dividing the number of cases of each outcome variable by the number of person-years. The associations between GPA and study outcomes are expressed as incidence rate ratios with 95% confidence intervals (CIs). We used the Kaplan-Meier method to construct estimated survival curves for the two calendar-time based sub-cohorts (i.e., 1992–2002 [early cohort] and 2003–2013 [recent cohort]).
Cox proportional hazard regression models were used to calculate hazard ratios (HRs) for total and sub-cohort follow-ups, after accounting for matched clusters (age, sex, and entry date). Our intermediate multivariate model adjusted for lifestyle factors (smoking, alcohol consumption, BMI), GP visits, and the CCI. The full multivariate model adjusted for all of the above as well as comorbidities and medications assessed prior to the index date. We then tested the significance of interaction between GPA and time period by adding the time period variable and the interaction terms to all models. We also calculated the absolute rate difference using an additive hazard model (23) for the two sub-cohort follow-ups and tested the additive interaction between GPA and time period
As previously described (24), our primary analysis used imputed missing values for covariates (i.e., smoking and alcohol use), employing a sequential regression method based on a set of covariates as predictors (IVEware for SAS, V.9.2; SAS Institute, Cary, North Carolina, USA). To minimize random error, we imputed five datasets and then combined estimates from these datasets (24). We calculated 95% CIs for all HRs. All p values were two-sided.
We used SAS, version 9.2 (SAS Institute, Cary, North Carolina, USA) for all statistical analyses.
RESULTS
Baseline Characteristics
Our study population included 465 patients with GPA and 4,613 patients without GPA. Table 1 shows baseline characteristics of patients in both groups. The average age of patients with GPA was 60.3 years (+/−14.6), comparable to other reports of GPA in the UK (18). Patients with GPA tended to have more comorbidities (including lower albumin levels) and greater use of cardiovascular medications, NSAIDs, and glucocorticoids compared with the comparison cohort. Over the course of the study, the number of patients enrolled in the THIN database increased, accounting for the increased number of GPA cases in the more recent cohort.
Table 1.
Baseline Characteristics According to the Presence of Granulomatosis with Polyangiitis
| Variables | Granulomatosis with Polyangiitis (n=465) |
Comparison Cohort (n=4613) |
P | |
|---|---|---|---|---|
| Age, years | 60.3 ± 14.6 | 60.3 ± 14.6 | 0.95 | |
| Sex | ||||
| Male | 245 (52.7%) | 2419 (52.4%) | 0.92 | |
| Female | 220 (47.3%) | 2194 (47.6%) | ||
| BMI (kg/m2) | 0.76 | |||
| Mean ± SD | 26.8 ± 5.1 | 26.9 ± 5.2 | ||
| <18.5 | 13 (2.8%) | 59 (1.3%) | ||
| 18.5–24.9 | 132 (28.4%) | 1390 (30.1%) | ||
| 25.0–29.9 | 145 (31.2%) | 1435 (31.1%) | ||
| ≥30.0 | 86 (18.5%) | 828 (17.9%) | ||
| Unknown | 89 (19.1%) | 901 (19.5%) | ||
|
Socio-Economic Deprivation Index Score |
2.6 ± 1.4 | 2.6 ± 1.4 | 0.78 | |
| Smoking | 0.01 | |||
| None | 229 (49.2%) | 2310 (50.1%) | ||
| Past | 128 (27.5%) | 993 (21.5%) | ||
| Current | 70 (15.1%) | 830 (18.0%) | ||
| Unknown | 38 (8.2%) | 480 (10.4%) | ||
| Alcohol | 0.58 | |||
| None | 72 (15.5%) | 663 (14.4%) | ||
| Past | 10 (2.2%) | 67 (1.5%) | ||
| Current | 298 (64.1%) | 2993 (64.9%) | ||
| Unknown | 85 (18.3%) | 890 (19.3%) | ||
| Charlson Comorbidity Index | 0.7 ± 1.2 | 0.3 ± 0.9 | <0.001 | |
| GP Visits | 6.1 ± 4.3 | 3.0 ± 3.5 | <0.001 | |
| Hypertension | 144 (31.0%) | 1303 (28.2%) | 0.22 | |
| Hyperlipidemia | 95 (20.4%) | 898 (19.5%) | 0.62 | |
| Stroke | 30 (6.5%) | 196 (4.2%) | 0.03 | |
| Ischemic Heart Disease | 45 (9.7%) | 403 (8.7%) | 0.50 | |
| Chronic Kidney Disease | 63 (13.5%) | 185 (4.0%) | <0.001 | |
| Diabetes | 24 (5.2%) | 326 (7.1%) | 0.12 | |
|
Angiotensin Converting Enzyme Inhibitor |
69 (14.8%) | 630 (13.7%) | 0.48 | |
| Aspirin | 74 (15.9%) | 627 (13.6%) | 0.17 | |
|
Angiotensin II Receptor Blockers |
21 (4.5%) | 218 (4.7%) | 0.84 | |
| Beta-Blockers | 69 (14.8%) | 599 (13.0%) | 0.26 | |
| Calcium Channel Blockers | 82 (17.6%) | 545 (11.8%) | <0.001 | |
| Diuretics | 111 (23.9%) | 714 (15.5%) | <0.001 | |
| NSAIDs | 160 (34.4%) | 846 (18.3%) | <0.001 | |
| Glucocorticoids | 182 (39.1%) | 203 (4.4%) | <0.001 | |
| Albumin, mmol/L | 37.0 ± 6.3 | 42.3 ± 3.7 | <.0001 | |
| Cholesterol, mmol/L | 5.0 ± 1.3 | 5.2 ± 1.1 | 0.03 | |
Data are represented as mean ± standard deviation (SD) or number (percentage); Socio-Economic Deprivation Index score was measured by the Townsend Deprivation Index, which was grouped into quintiles from 1 (least deprived) to 5 (most deprived); Hyperlipidemia is defined as having either a diagnosis of hyperlipidemia or a prescription for antihyperlipidemics; Glucocorticoids are oral or injectable steroids; Diuretics are loop or thiazide diuretics.
All-Cause Mortality During the Entire Follow-Up Period (1992–2013)
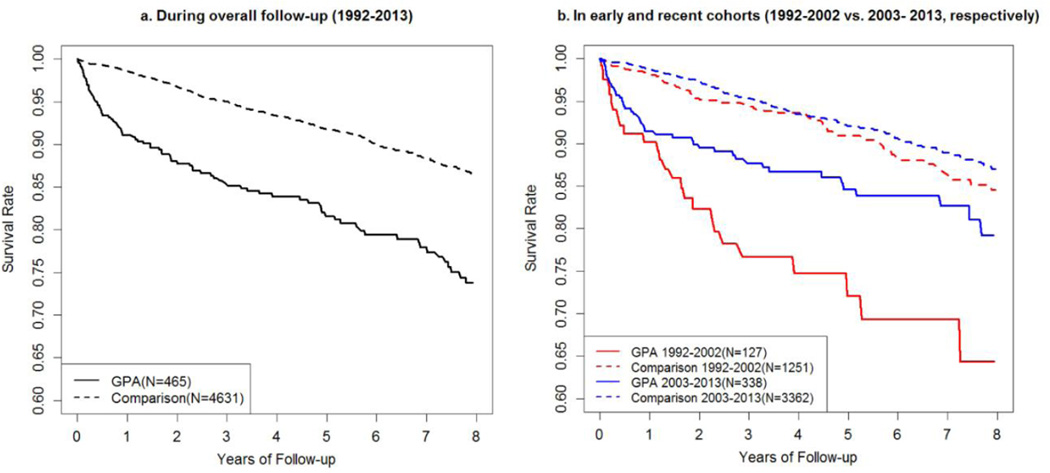
There were 101 deaths in the GPA group (N=465) compared to 511 in the comparison cohort (N=4613) (Table 2). The incidence rate of death (per 1000 person-years) in the GPA group was 43.5 (95% CI, 35.4–52.9) compared to 19.3 (95% CI, 17.6–21.0) in the comparison cohort (Figure 1a). The age-, sex-, and entry time-matched HR for mortality among GPA patients was 2.76 (95% CI, 2.19–3.47). The HR for mortality remained significant, after adjusting for potential confounders (2.52; 95%, 1.91–3.32).
Table 2.
Incidence Rates and Hazard Ratios (HR) for Associations Between Granulomatosis with Polyangiitis (GPA) and Death (1992–2013)
| GPA Status |
N | Deaths | Follow- up Time (person- years) |
Mean Follow- up (years) |
Incidence Rate (cases per 1000 person- years) |
Age, Sex, and Entry- time Matched HR (95%CI)* |
+ GPs visits, BMI, SDI, Smoking, Alcohol, and Charlson Index Adjusted HR (95%CI) |
+ Comorbidity and Medicines Adjusted HR* (95%CI) |
|
|---|---|---|---|---|---|---|---|---|---|
| Total | Yes | 465 | 101 | 2321.5 | 5.0 | 43.51 (35.44 to 52.86) |
2.76 (2.19 to 3.47) |
2.64 (2.07 to 3.38) |
2.52 (1.91 to 3.32) |
| No | 4613 | 511 | 26521.4 | 5.7 | 19.27 (17.63 to 21.01) |
1.0 (reference) |
1.0 (reference) | 1.0 (reference) |
|
| Female | Yes | 220 | 38 | 1182.7 | 5.4 | 32.13 (22.74 to 44.10) |
2.20 (1.54 to 3.16) |
2.31 (1.57 to 3.39) |
2.22 (1.45 to 3.38) |
| No | 2194 | 235 | 13097.2 | 6.0 | 17.94 (15.72 to 20.39) |
1.0 (reference) |
1.0 (reference) | 1.0 (reference) |
|
| Male | Yes | 245 | 63 | 1138.9 | 4.6 | 55.32 (42.51 to 70.77) |
3.27 (2.42 to 4.41) |
2.86 (2.06 to 3.96) |
2.87 (1.96 to 4.20) |
| No | 2419 | 276 | 13424.2 | 5.5 | 20.56 (18.21 to 23.13) |
1.0 (reference) |
1.0 (reference) | 1.0 (reference) |
|
|
Age ≤ 65 |
Yes | 275 | 32 | 1603.7 | 5.8 | 19.95 (13.65 to 28.17) |
3.13 (2.07 to 4.72) |
2.75 (1.76 to 4.30) |
2.52 (1.51 to 4.19) |
| No | 2730 | 116 | 16683.1 | 6.1 | 6.95 (5.75 to 8.34) |
1.0 (reference) |
1.0 (reference) | 1.0 (reference) |
|
|
Age > 65 |
Yes | 190 | 69 | 717.9 | 3.8 | 96.11 (74.78 to 121.64) |
2.61 (1.98 to 3.44) |
2.57 (1.91 to 3.46) |
2.37 (1.69 to 3.34) |
| No | 1883 | 395 | 9838.3 | 5.2 | 40.15 (36.29 to 44.31) |
1.0 (reference) |
1.0 (reference) | 1.0 (reference) |
|
Adjusted for all of the covariates in Table 1
Figure 1.
Survival According to the Presence of Granulomatosis with Polyangiitis
Overall, the HR of death was similar among male and female patients in the fully adjusted models. Among female patients the HR was 2.22 (95%, 1.45–3.38) compared to 2.87 (95%, 1.96–4.20) in male patients. Similarly, there was no apparent difference in the mortality HR between those older and younger than 65 years of age. Patients over 65 years old had a HR of 2.37 (95%, 1.69–3.34) compared to 2.52 (95% 1.51–4.19) among younger patients.
With regard to the timing of death, patients with GPA were at the greatest risk of death in the first year following diagnosis (Figure 1a, Table 3). The incidence rate for death in the first year was 97.4 (96% CI, 69.6–132.6) per 1000 person-years compared to 42.5 (95% CI, 34.2–52.1) over the ten years following diagnosis. In the fully-adjusted model, the HR for mortality in the first year was 6.31 (95% CI, 3.62–10.98) compared to 2.51 (95% CI, 1.88–3.36) over the ten years following diagnosis. Although still elevated, the risk of death of GPA declined towards that of the general population in the years following diagnosis.
Table 3.
Incidence Rates and Hazard Ratios (HR) for Associations Between Granulomatosis with Polyangiitis (GPA) and Death According to Different Follow Up Time Period
| GPA Status |
N | Deaths | Follow- up Time (person- years) |
Mean Follow- up (years) |
Incidence Rate (cases per 1000 person- years) |
Age, Sex, and Entry- time Matched HR (95%CI)* |
+ GPs visits, BMI, SDI, Smoking, Alcohol, and Charlson Index Adjusted HR (95%CI) |
+ Comorbidity and Medicines Adjusted HR* (95%CI) |
|
|---|---|---|---|---|---|---|---|---|---|
| Overall | Yes | 465 | 101 | 2321.5 | 5.0 | 43.51 (35.44 to 52.86) |
2.76 (2.19 to 3.47) |
2.64 (2.07 to 3.38) |
2.52 (1.91 to 3.32) |
| No | 4613 | 511 | 26521.4 | 5.7 | 19.27 (17.63 to 21.01) |
1.0 (reference) |
1.0 (reference) | 1.0 (reference) |
|
|
0–1 year |
Yes | 465 | 40 | 410.7 | 0.9 | 97.39 (69.58 to 132.62) |
6.67 (4.44 to 10.00) |
6.85 (4.21 to 11.15) |
6.31 (3.62 to 10.98) |
| No | 4613 | 62 | 4344.8 | 0.9 | 14.27 (10.94 to 18.29) |
1.0 (reference) |
1.0 (reference) | 1.0 (reference) |
|
|
0–3 years |
Yes | 465 | 61 | 1050.8 | 2.3 | 58.05 (44.40 to 74.57) |
3.66 (2.72 to 4.94) |
3.54 (2.56 to 4.90) |
3.35 (2.31 to 4.85) |
| No | 4613 | 196 | 11477.0 | 2.5 | 17.08 (14.77 to 19.64) |
1.0 (reference) |
1.0 (reference) | 1.0 (reference) |
|
|
0–5 years |
Yes | 465 | 72 | 1531.2 | 3.3 | 47.02 (36.79 to 59.22) |
3.03 (2.32 to 3.97) |
2.94 (2.21 to 3.92) |
2.78 (2.01 to 3.84) |
| No | 4613 | 290 | 16702.2 | 3.6 | 17.36 (15.42 to 19.48) |
1.0 (reference) |
1.0 (reference) | 1.0 (reference) |
|
|
0–10 years |
Yes | 465 | 91 | 2143.5 | 4.6 | 42.45 (34.18 to 52.12) |
2.66 (2.10 to 3.38) |
2.61 (2.02 to 3.37) |
2.51 (1.88 to 3.36) |
| No | 4613 | 442 | 23807.0 | 5.2 | 18.57 (16.88 to 20.38) |
1.0 (reference) |
1.0 (reference) | 1.0 (reference) |
|
Adjusted for all of the covariates in Table 1
Mortality Trends Over Calendar-Time
Figure 1b shows the survival rate of patients during the follow up according to the calendar-year based sub-cohort. The early cohort (1992–2002) GPA patients had considerably higher mortality rates than the recent cohort (2003–2013) patients (72.0 vs. 35.7 cases per 1000 person-years; Table 4), as compared with a moderate improvement in mortality rates in the comparison cohorts between the two periods (19.8 vs 17.0 cases per 1000 person-years). The corresponding absolute mortality rate difference was 52.2 (95% CI, 25.1–79.2) cases and 18.7 (95% CI, 8.3–29.1) cases per 1000 person-years (p for interaction=0.025). When matched for age, sex, and entry-time, the HR for mortality was 4.34 (95%, 2.72–6.92) in the early cohort in contrast to 2.41 (95%, 1.74–3.34) in the recent cohort (p for interaction = 0.043). In the intermediate multivariate model adjusted for lifestyle factors (smoking, alcohol consumption, BMI), SDI, GP visits, and the CCI, the HR remained elevated in the early cohort (3.73, 95%, 2.11–6.61) compared to that of the recent cohort (2.51, 95%, 1.75–3.59). In the fully adjusted multivariate model adjusting for comorbidities and medications, the HR was 3.54 (95%, 1.73–7.24) in the early cohort and 2.46 (95%, 1.67–3.64) in the recent cohort.
Table 4.
Incidence Rates and Hazard Ratios (HR) for Associations Between Granulomatosis with Polyangiitis (GPA) and Death According to Calendar Time-Based Subgroups (1992–2002 vs. 2003–2013)
| GPA Status |
N | Deaths | Follow- up Time (person- years) |
Mean Follow- up (years) |
Incidence Rate (cases per 1000 person-years) |
Age, Sex, and Entry- time Matched HR (95%CI)* |
+ GPs visits, BMI, SDI, Smoking, Alcohol, and Charlson Index Adjusted HR (95%CI) |
+ Comorbidity and Medicines Adjusted HR* (95%CI) |
|
|---|---|---|---|---|---|---|---|---|---|
| Total | Yes | 465 | 101 | 2321.5 | 5.0 | 43.51 (35.44 to 52.86) |
2.76 (2.19 to 3.47) |
2.64 (2.07 to 3.38) |
2.52 (1.91 to 3.32) |
| No | 4613 | 511 | 26521.4 | 5.7 | 19.27 (17.63 to 21.01) |
1.0 (reference) |
1.0 (reference) | 1.0 (reference) |
|
|
1992– 2002 |
Yes | 127 | 27 | 374.9 | 3.0 | 72.02 (47.46 to 104.78) |
4.34 (2.72 to 6.92) |
3.73 (2.11 to 6.61) |
3.54 (1.73 to 7.24) |
| No | 1251 | 85 | 4296.5 | 3.4 | 19.78 (15.80 to 24.46) |
1.0 (reference) |
1.0 (reference) | 1.0 (reference) |
|
|
2003– 2013 |
Yes | 338 | 47 | 1316.4 | 3.9 | 35.70 (26.23 to 47.48) |
2.41 (1.74 to 3.34) |
2.51 (1.75 to 3.59) |
2.46 (1.67 to 3.64) |
| No | 3362 | 244 | 14339.7 | 4.3 | 17.02 (14.95 to 19.29) |
1.0 (reference) |
1.0 (reference) | 1.0 (reference) |
|
Adjusted for all of the covariates in Table 1
DISCUSSION
Granulomatosis with polyangiitis (GPA) is a severe, life threatening form of small-vessel vasculitis previously associated with increased mortality due to the disease itself as well as due to complications of treatment (6, 10–13, 18). In this large general practice cohort representative of the UK population, we confirm the observation that GPA is associated with increased mortality, especially in the first year following diagnosis. Furthermore, the survival of GPA patients has improved considerably over the past two decades, although the mortality in the recent cohort (2003–2013) was still higher than that in non-GPA patients. These trends were evident in both ratio and difference measures, as compared with the background mortality improvement over the years. These findings provide the first population-based evidence for the benefits of improved GPA care over the past two decades. A prior study by Takala et al found no difference over time when examining the two decades spanning 1981–2000 (7). While these data are encouraging, our data also indicate a notably increased risk of premature mortality in GPA patients even over the past decade.
Our findings suggest that trends in the management of GPA that have emphasized less cumulative cyclophosphamide exposure and greater use of azathioprine and other steroid-sparing agents may have had a favorable impact on survival. Prior reports have described an increased risk of cardiovascular disease and malignancy in patients with GPA (3, 25, 26); thus, advances in the management of these conditions may also account for some of the increased survival we observed in the later cohort. Other secular trends in the management of infectious, renal, and pulmonary complications – of which ANCA patients are at an elevated risk – may have also influenced the trend in mortality. Our results suggest that differences in mortality between the recent and early cohorts may be driven more by improved survival after the first year of diagnosis (Figure 1b). This may support our hypothesis that shifts in management might drive the improved survival since many of the complications of treatment (e.g., infection, secondary malignancy) occur after prolonged exposure. Additionally, improvements in the management of complications (e.g., infection, renal disease) may impact late survival. Yet, the remaining increased mortality risk, especially early in the disease course, calls for further improvement in GPA care.
The overall adjusted HR of mortality in our cohort appears to be somewhat lower (2.5; 95% CI, 1.90–3.28) than nearly all prior reports (2–9). One explanation for this is the nature of the THIN database, which includes diagnoses made in both inpatient and outpatient settings, thus enriching our dataset with patients of varying disease severity. Another explanation is the inclusion of patients diagnosed after 2004, a period characterized by improved survival which has not been evaluated in previous studies. Indeed, the mortality rate in the early cohort is very similar to that reported by Takala et al. in their retrospective (1981–2000) cohort study using a Finnish hospital discharge registry (7); that study used age- and sex-matched controls from the same practice as the case. Furthermore, although we used person-time based mortality rates (i.e., a preferred option in observational studies such as ours) as our risk estimate, the proportion of deaths during our early cohort follow-up closely agreed with a previous UK GP data-based study (9) whose period (1989–2004) coincided well with our early cohort follow-up time (1992–2002). That previous UK study showed 21% deaths in the GPA cohort vs. 7.8% in the control cohort, whereas the corresponding proportions in our study were 21% and 7%, respectively (calculated based on the data in Table 4). Nevertheless, we did not observe any significant difference in the mortality rate among male and female patients unlike prior reports (4, 7, 8, 11, 13, 27). Similarly, being over the age of 65 years did not confer a significantly increased risk of death in our cohort, which contrasts with previous observations (8, 27).
Our study has several strengths. It is one of the largest studies evaluating mortality due to GPA in a population-based cohort and includes patients diagnosed after 2004, a period not previously studied. The diagnosis and management of GPA has changed significantly between 1992 and 2013 with a wider availability of ANCA testing to assist with diagnosis (28) and trends toward lower cumulative cyclophosphamide doses and the increased use of conventional steroid-sparing agents such as azathioprine and methotrexate for maintenance therapy (14–18). The extended period covered in this study allowed us to evaluate mortality in the setting of these trends in management. In contrast to Takala et al. (7), the inclusion of GPA cases diagnosed in our general population context limited bias towards the more severe cases typically admitted to hospitals and makes our findings more generalizable. The relative recent approval in the UK of rituximab for remission induction in GPA means that the impact of this new treatment could not be evaluated in this study.
Despite these strengths, our study has limitations. Our study relied on physician diagnosis of GPA, which was not confirmed according to pre-specified criteria. However, prior studies have confirmed the validity of Read codes for GPA according to the American College of Rheumatology criteria and the Chapel Hill Consensus Conference definition (19). We did not have sufficient detail on the cause of death in a number of cases in our dataset, precluding investigations on the cause of death in this cohort. Nevertheless, the overall all-cause mortality trends are critically important in their own right. Future studies should address potential specific reasons behind this improvement, including changes in specific therapeutics (including the recent use of rituximab (29)), the data of which are generally incomplete in our GP practice-based database.
CONCLUSIONS
In conclusion, we found that mortality is increased among individuals with GPA, even in the most recent cohort (2003–2013). Further, our findings provide the first population-based evidence for a considerable trend toward improved survival over the past two decades. These data affirm the benefits of recent trends in the management of GPA and its complications.
Acknowledgments
None
Funding info: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: None
Contributorship: All authors contributed to the following: substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethical approval information: This study was approved by the THIN Scientific Review Committee IRB.
Data sharing statement: At this moment there are no additional unpublished data.
REFERENCES
- 1.Falk RJ, Gross WL, Guillevin L, Hoffman G, Jayne DR, Jennette JC, et al. Granulomatosis with polyangiitis (Wegener's): an alternative name for Wegener's granulomatosis. Ann Rheum Dis. 2011 Apr;70(4):704. doi: 10.1136/ard.2011.150714. [DOI] [PubMed] [Google Scholar]
- 2.Mohammad AJ, Jacobsson LT, Westman KW, Sturfelt G, Segelmark M. Incidence and survival rates in Wegener's granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome and polyarteritis nodosa. Rheumatology (Oxford) 2009 Dec;48(12):1560–1565. doi: 10.1093/rheumatology/kep304. [DOI] [PubMed] [Google Scholar]
- 3.Flossmann O, Berden A, de Groot K, Hagen C, Harper L, Heijl C, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis. 2011 Mar;70(3):488–494. doi: 10.1136/ard.2010.137778. [DOI] [PubMed] [Google Scholar]
- 4.Matteson EL, Gold KN, Bloch DA, Hunder GG. Long-term survival of patients with Wegener's granulomatosis from the American College of Rheumatology Wegener's Granulomatosis Classification Criteria Cohort. Am J Med. 1996 Aug;101(2):129–134. doi: 10.1016/s0002-9343(96)80066-0. [DOI] [PubMed] [Google Scholar]
- 5.Booth AD, Almond MK, Burns A, Ellis P, Gaskin G, Neild GH, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis. 2003 Apr;41(4):776–784. doi: 10.1016/s0272-6386(03)00025-8. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson P, Jacobsson L, Lindell A, Nilsson JA, Skogh T. Improved outcome in Wegener's granulomatosis and microscopic polyangiitis? A retrospective analysis of 95 cases in two cohorts. J Intern Med. 2009 Apr;265(4):496–506. doi: 10.1111/j.1365-2796.2008.02060.x. [DOI] [PubMed] [Google Scholar]
- 7.Takala JH, Kautiainen H, Leirisalo-Repo M. Survival of patients with Wegener's granulomatosis diagnosed in Finland in 1981–2000. Scand J Rheumatol. 2010;39(1):71–76. doi: 10.3109/03009740903140701. [DOI] [PubMed] [Google Scholar]
- 8.Lane SE, Watts RA, Shepstone L, Scott DG. Primary systemic vasculitis: clinical features and mortality. QJM. 2005 Feb;98(2):97–111. doi: 10.1093/qjmed/hci015. [DOI] [PubMed] [Google Scholar]
- 9.Luqmani R, Suppiah R, Edwards CJ, Phillip R, Maskell J, Culliford D, et al. Mortality in Wegener's granulomatosis: a bimodal pattern. Rheumatology (Oxford) 2011 Apr;50(4):697–702. doi: 10.1093/rheumatology/keq351. [DOI] [PubMed] [Google Scholar]
- 10.Fauci AS, Wolff SM. Wegener's granulomatosis: studies in eighteen patients and a review of the literature. Medicine (Baltimore) 1973 Nov;52(6):535–561. doi: 10.1097/00005792-197311000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992 Mar 15;116(6):488–498. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 12.Faurschou M, Sorensen IJ, Mellemkjaer L, Loft AG, Thomsen BS, Tvede N, et al. Malignancies in Wegener's granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patients. J Rheumatol. 2008 Jan;35(1):100–105. [PubMed] [Google Scholar]
- 13.Holle JU, Gross WL, Latza U, Nolle B, Ambrosch P, Heller M, et al. Improved outcome in 445 patients with Wegener's granulomatosis in a German vasculitis center over four decades. Arthritis Rheum. 2011 Jan;63(1):257–266. doi: 10.1002/art.27763. [DOI] [PubMed] [Google Scholar]
- 14.de Groot K, Adu D, Savage CO EUVAS (European vasculitis study group) The value of pulse cyclophosphamide in ANCA-associated vasculitis: meta-analysis and critical review. Nephrol Dial Transplant. 2001 Oct;16(10):2018–2027. doi: 10.1093/ndt/16.10.2018. [DOI] [PubMed] [Google Scholar]
- 15.Langford CA, Talar-Williams C, Barron KS, Sneller MC. A staged approach to the treatment of Wegener's granulomatosis: induction of remission with glucocorticoids and daily cyclophosphamide switching to methotrexate for remission maintenance. Arthritis Rheum. 1999 Dec;42(12):2666–2673. doi: 10.1002/1529-0131(199912)42:12<2666::AID-ANR24>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 16.Langford CA, Talar-Williams C, Barron KS, Sneller MC. Use of a cyclophosphamide-induction methotrexate-maintenance regimen for the treatment of Wegener's granulomatosis: extended follow-up and rate of relapse. Am J Med. 2003 Apr 15;114(6):463–469. doi: 10.1016/s0002-9343(03)00077-9. [DOI] [PubMed] [Google Scholar]
- 17.Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniene J, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003 Jul 3;349(1):36–44. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 18.Langford CA. Wegener's granulomatosis: current and upcoming therapies. Arthritis Res Ther. 2003;5(4):180–191. doi: 10.1186/ar771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watts RA, Al-Taiar A, Scott DG, Macgregor AJ. Prevalence and incidence of Wegener's granulomatosis in the UK general practice research database. Arthritis Rheum. 2009 Oct 15;61(10):1412–1416. doi: 10.1002/art.24544. [DOI] [PubMed] [Google Scholar]
- 20.Simon C. Overview of the GP Contract. InnovAit. 2008;1:134. [Google Scholar]
- 21.Chisholm J. The Read clinical classification. BMJ. 1990 Apr 28;300(6732):1092. doi: 10.1136/bmj.300.6732.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Rod NH, Lange T, Andersen I, Marott JL, Diderichsen F. Additive interaction in survival analysis: use of the additive hazards model. Epidemiology. 2012 Sep;23(5):733–737. doi: 10.1097/EDE.0b013e31825fa218. [DOI] [PubMed] [Google Scholar]
- 24.Dubreuil M, Rho YH, Man A, Zhu Y, Zhang Y, Love TJ, et al. Diabetes incidence in psoriatic arthritis, psoriasis and rheumatoid arthritis: a UK population-based cohort study. Rheumatology (Oxford) 2014 Feb;53(2):346–352. doi: 10.1093/rheumatology/ket343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suppiah R, Judge A, Batra R, Flossmann O, Harper L, Hoglund P, et al. A model to predict cardiovascular events in patients with newly diagnosed Wegener's granulomatosis and microscopic polyangiitis. Arthritis Care Res (Hoboken) 2011 Apr;63(4):588–596. doi: 10.1002/acr.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heijl C, Harper L, Flossmann O, Stucker I, Scott DG, Watts RA, et al. Incidence of malignancy in patients treated for antineutrophil cytoplasm antibody-associated vasculitis: follow-up data from European Vasculitis Study Group clinical trials. Ann Rheum Dis. 2011 Aug;70(8):1415–1421. doi: 10.1136/ard.2010.145250. [DOI] [PubMed] [Google Scholar]
- 27.Mukhtyar C, Flossmann O, Hellmich B, Bacon P, Cid M, Cohen-Tervaert JW, et al. Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League Against Rheumatism systemic vasculitis task force. Ann Rheum Dis. 2008 Jul;67(7):1004–1010. doi: 10.1136/ard.2007.071936. [DOI] [PubMed] [Google Scholar]
- 28.Niles JL, Pan GL, Collins AB, Shannon T, Skates S, Fienberg R, et al. Antigen-specific radioimmunoassays for anti-neutrophil cytoplasmic antibodies in the diagnosis of rapidly progressive glomerulonephritis. J Am Soc Nephrol. 1991 Jul;2(1):27–36. doi: 10.1681/ASN.V2127. [DOI] [PubMed] [Google Scholar]
- 29.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010 Jul 15;363(3):221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]