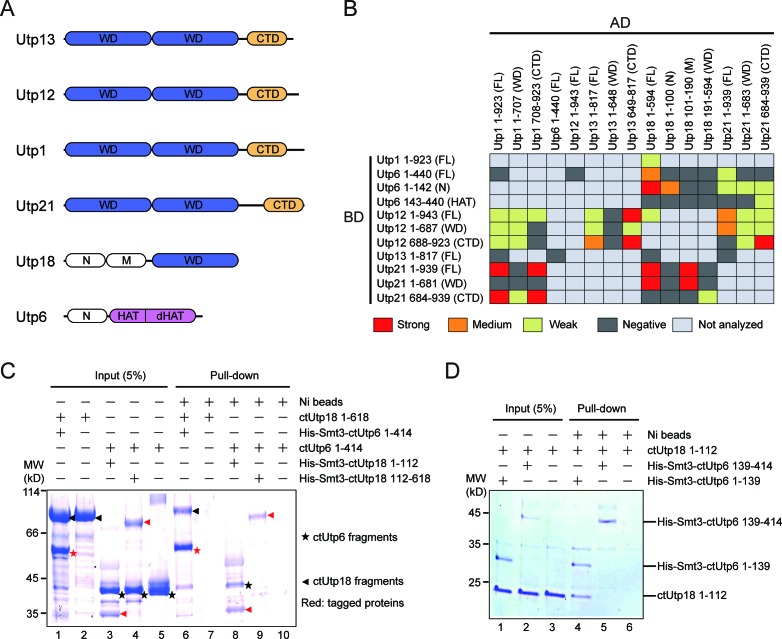
Figure 1.
Domain–domain interaction of UTPB proteins. (A) Domain diagrams of UTPB proteins. Utp13, Utp12, Utp1 and Utp21 are composed of tandem WD domains and a C-terminal domain (CTD). Utp18 is composed of an N, M and WD domain. Utp6 is composed of an N, HAT and deviant HAT (dHAT) domain. (B) Two-hybrid interactions among UTPB protein fragments. The indicated Saccharomyces cerevisiae genes were fused to the activation domain (AD) and GAL4 DNA-binding domain (BD) and co-expressed in the AH109 strain. An interaction was identified as weak, medium or strong when the two-hybrid strain is able to grown on SC medium lacking Leu, Trp and His (low stringency), or the above medium additionally containing 5 mM 3- AT (medium stringency) or lacking Ade (high stringency). Negative interaction indicates no growth on the low stringency medium. FL, full-length. The previously published interactions between Utp21 and Utp18 are included for completeness (22). (C and D) Pull-down assay of ctUtp6 and ctUtp18. One protein contained a His-Smt3-tag and was pulled down with Ni Sepharose beads. The tagged proteins were marked with red symbol in C. The input (5%) and bound proteins were analyzed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie staining. The positions of molecular weight markers are indicated on the left.