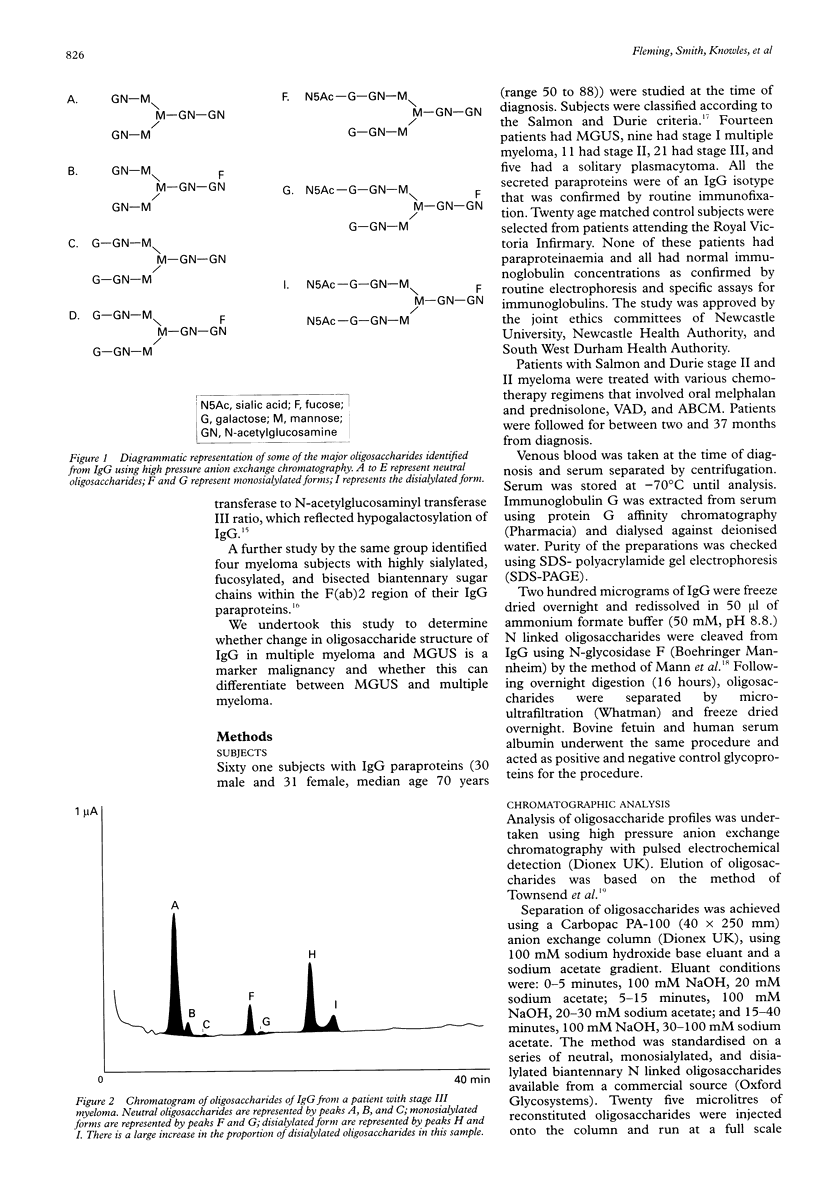
Abstract
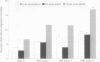
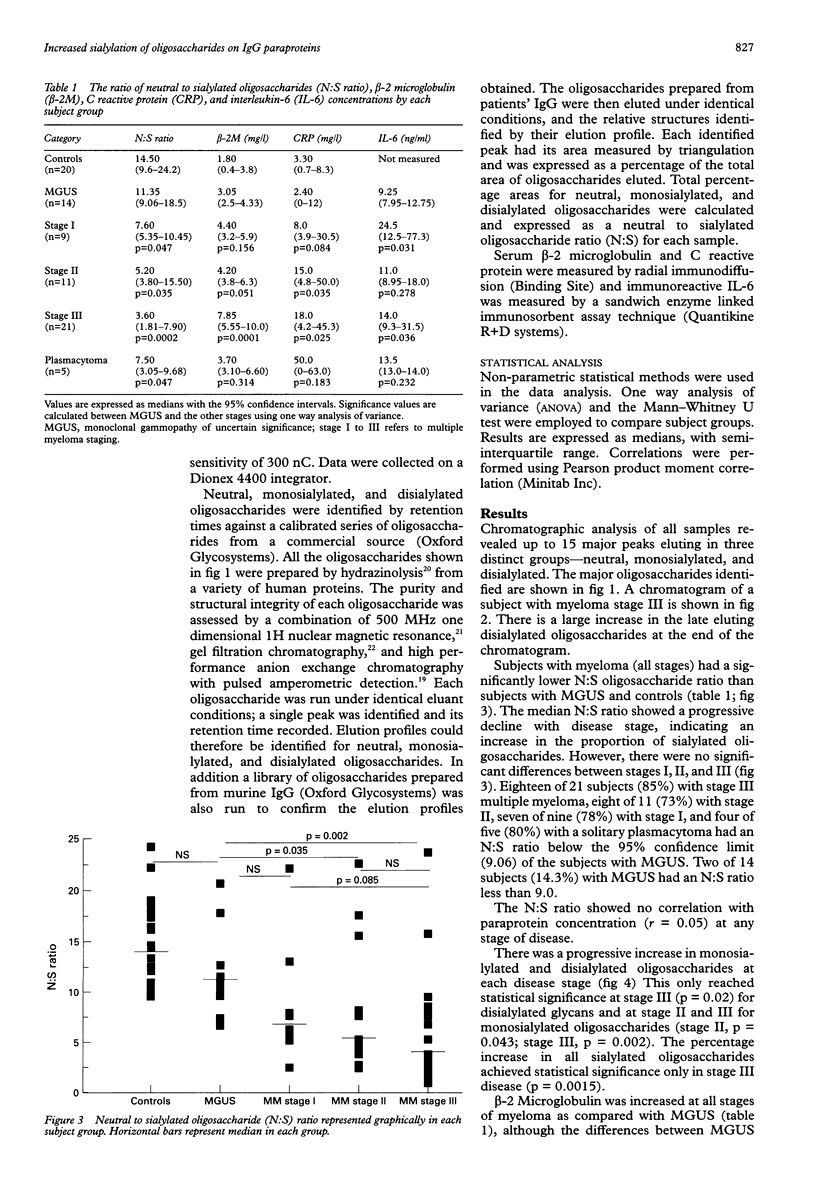
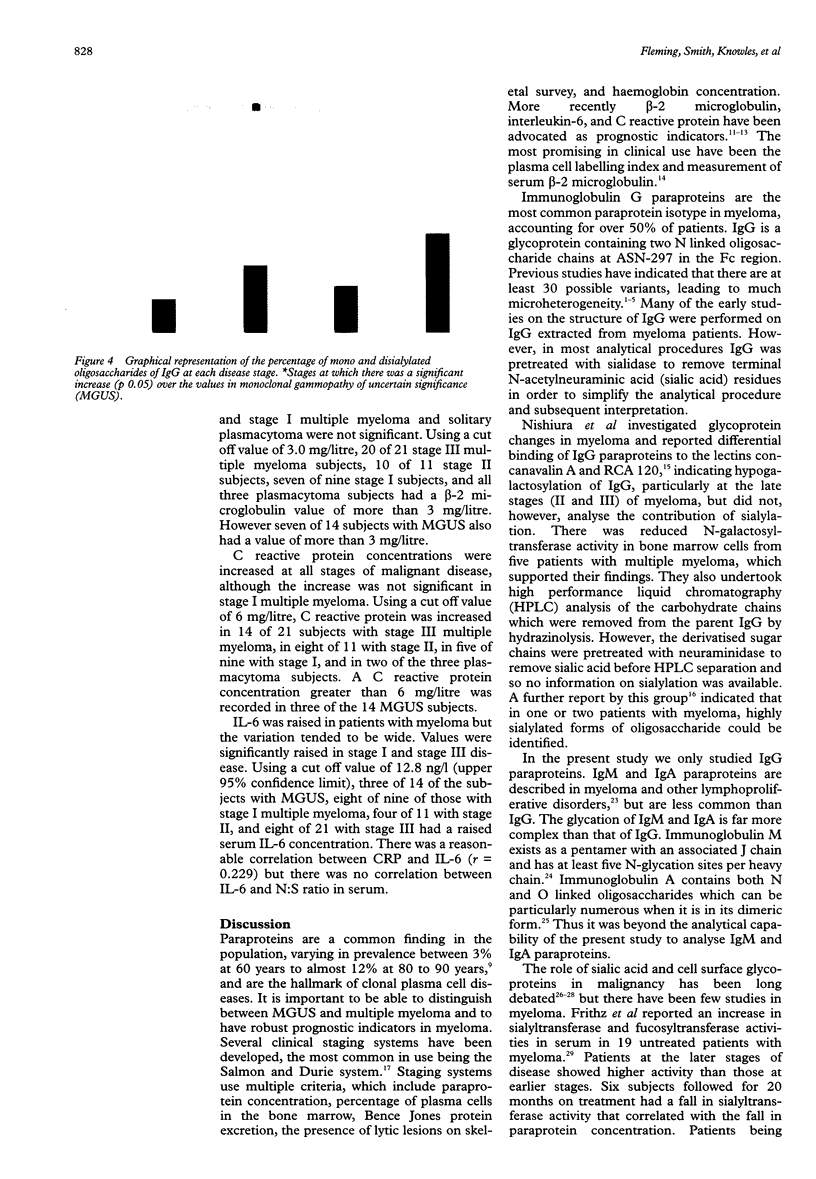
AIMS: To investigate whether changes in carbohydrate structure of IgG are related to malignancy and stage of disease in myeloma and monoclonal gammopathy of uncertain significance (MGUS). METHODS: 61 patients were studied at diagnosis: 14 with MGUS, nine with stage I multiple myeloma, 11 with stage II, 21 with stage III, and five with solitary plasmacytoma. IgG was extracted from serum by protein G affinity chromatography. Oligosaccharides were cleaved from the protein backbone enzymatically by N-glycosidase F. Oligosaccharide analysis was performed by high pressure anion exchange chromatography with pulsed electrochemical detection (HPAE-PED). RESULTS: Up to 15 oligosaccharide peaks were identified in three major fractions: neutral, monosialylated, and disialylated. Patients with myeloma showed an increase in the proportion of sialylated oligosaccharides in comparison with patients with MGUS. The ratio of neutral to sialylated oligosaccharides (N:S) was reduced at all stages of myeloma compared with MGUS: MGUS, 11.35; myeloma stage I, 7.6 (p = 0.047); stage II, 5.20 (p = 0.035); stage III, 3.60 (p = 0.0002); plasmacytoma, 7.5 (p = 0.046). The N:S ratio was independent of paraprotein concentration (r = 0.05). CONCLUSIONS: The ratio of neutral to sialylated oligosaccharides may act as a new marker of malignancy in IgG paraproteinaemia and warrants further investigation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altevogt P., Fogel M., Cheingsong-Popov R., Dennis J., Robinson P., Schirrmacher V. Different patterns of lectin binding and cell surface sialylation detected on related high- and low-metastatic tumor lines. Cancer Res. 1983 Nov;43(11):5138–5144. [PubMed] [Google Scholar]
- Anderson D. R., Atkinson P. H., Grimes W. J. Major carbohydrate structures at five glycosylation sites on murine IgM determined by high resolution 1H-NMR spectroscopy. Arch Biochem Biophys. 1985 Dec;243(2):605–618. doi: 10.1016/0003-9861(85)90538-7. [DOI] [PubMed] [Google Scholar]
- Axelsson U., Bachmann R., Hällén J. Frequency of pathological proteins (M-components) om 6,995 sera from an adult population. Acta Med Scand. 1966 Feb;179(2):235–247. doi: 10.1111/j.0954-6820.1966.tb05453.x. [DOI] [PubMed] [Google Scholar]
- CLAMP J. R., PUTNAM F. W. THE CARBOHYDRATE PROSTHETIC GROUP OF HUMAN GAMMA-GLOBULIN. J Biol Chem. 1964 Oct;239:3233–3240. [PubMed] [Google Scholar]
- Cohen A. M., Allalouf D., Bessler H., Djaldetti M., Malachi T., Levinsky H. Sialyltransferase activity and sialic acid levels in multiple myeloma and monoclonal gammopathy. Eur J Haematol. 1989 Mar;42(3):289–292. doi: 10.1111/j.1600-0609.1989.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Cohen A. M., Allalouf D., Djaldetti M., Weigl K., Lehrer N., Levinsky H. Sialyltransferase activity in plasma cells of multiple myeloma. Eur J Haematol. 1989 Sep;43(3):191–194. doi: 10.1111/j.1600-0609.1989.tb00281.x. [DOI] [PubMed] [Google Scholar]
- Cohen A. M., Wacks T., Lurie B. B., Levinsky H. Interferon-alpha and dexamethasone effect on AF10 myeloma cell line sialytransferase activity. Biochem Med Metab Biol. 1993 Aug;50(1):9–17. doi: 10.1006/bmmb.1993.1042. [DOI] [PubMed] [Google Scholar]
- Durie B. G. Staging and kinetics of multiple myeloma. Semin Oncol. 1986 Sep;13(3):300–309. [PubMed] [Google Scholar]
- Frithz G., Ronquist G., Ericsson P. Serum sialyltransferase and fucosyltransferase activities in patients with multiple myeloma. Eur J Cancer Clin Oncol. 1985 Aug;21(8):913–917. doi: 10.1016/0277-5379(85)90107-5. [DOI] [PubMed] [Google Scholar]
- Fujii S., Nishiura T., Nishikawa A., Miura R., Taniguchi N. Structural heterogeneity of sugar chains in immunoglobulin G. Conformation of immunoglobulin G molecule and substrate specificities of glycosyltransferases. J Biol Chem. 1990 Apr 15;265(11):6009–6018. [PubMed] [Google Scholar]
- Kinoshita N., Ohno M., Nishiura T., Fujii S., Nishikawa A., Kawakami Y., Uozumi N., Taniguchi N. Glycosylation at the Fab portion of myeloma immunoglobulin G and increased fucosylated biantennary sugar chains: structural analysis by high-performance liquid chromatography and antibody-lectin enzyme immunoassay using Lens culinaris agglutinin. Cancer Res. 1991 Nov 1;51(21):5888–5892. [PubMed] [Google Scholar]
- Kobata A., Yamashita K., Takasaki S. BioGel P-4 column chromatography of oligosaccharides: effective size of oligosaccharides expressed in glucose units. Methods Enzymol. 1987;138:84–94. doi: 10.1016/0076-6879(87)38008-5. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Keller J., Baenziger J., Kornfeld S. The structure of the glycopeptide of human gamma G myeloma proteins. J Biol Chem. 1971 May 25;246(10):3259–3268. [PubMed] [Google Scholar]
- Koyama S., Daiyasu H., Hase S., Kobayashi Y., Kyogoku Y., Ikenaka T. 1H-NMR analysis of the sugar structures of glycoproteins as their pyridylamino derivatives. FEBS Lett. 1986 Dec 15;209(2):265–268. doi: 10.1016/0014-5793(86)81125-5. [DOI] [PubMed] [Google Scholar]
- Kyle R. A. Monoclonal gammopathy and multiple myeloma in the elderly. Baillieres Clin Haematol. 1987 Jun;1(2):533–557. doi: 10.1016/s0950-3536(87)80012-4. [DOI] [PubMed] [Google Scholar]
- Kyle R. A. Why better prognostic factors for multiple myeloma are needed. Blood. 1994 Apr 1;83(7):1713–1716. [PubMed] [Google Scholar]
- Mackiewicz A., Kushner I. Interferon beta 2/B-cell stimulating factor 2/interleukin 6 affects glycosylation of acute phase proteins in human hepatoma cell lines. Scand J Immunol. 1989 Mar;29(3):265–271. doi: 10.1111/j.1365-3083.1989.tb01124.x. [DOI] [PubMed] [Google Scholar]
- Nakao H., Nishikawa A., Karasuno T., Nishiura T., Iida M., Kanayama Y., Yonezawa T., Tarui S., Taniguchi N. Modulation of N-acetylglucosaminyltransferase III, IV and V activities and alteration of the surface oligosaccharide structure of a myeloma cell line by interleukin 6. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1260–1266. doi: 10.1016/0006-291x(90)91585-g. [DOI] [PubMed] [Google Scholar]
- Nakao H., Nishikawa A., Nishiura T., Kanayama Y., Tarui S., Taniguchi N. Hypogalactosylation of immunoglobulin G sugar chains and elevated serum interleukin 6 in Castleman's disease. Clin Chim Acta. 1991 Mar 29;197(3):221–228. doi: 10.1016/0009-8981(91)90142-y. [DOI] [PubMed] [Google Scholar]
- Niesvizky R., Siegel D., Michaeli J. Biology and treatment of multiple myeloma. Blood Rev. 1993 Mar;7(1):24–33. doi: 10.1016/0268-960x(93)90021-u. [DOI] [PubMed] [Google Scholar]
- Nishiura T., Fujii S., Kanayama Y., Nishikawa A., Tomiyama Y., Iida M., Karasuno T., Nakao H., Yonezawa T., Taniguchi N. Carbohydrate analysis of immunoglobulin G myeloma proteins by lectin and high performance liquid chromatography: role of glycosyltransferases in the structures. Cancer Res. 1990 Sep 1;50(17):5345–5350. [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985 Aug 1;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- Parekh R., Isenberg D., Rook G., Roitt I., Dwek R., Rademacher T. A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J Autoimmun. 1989 Apr;2(2):101–114. doi: 10.1016/0896-8411(89)90148-0. [DOI] [PubMed] [Google Scholar]
- Pierce-Cretel A., Pamblanco M., Strecker G., Montreuil J., Spik G., Dorland L., Van Halbeek H., Vliegenthart J. F. Primary structure of the N-glycosidically linked sialoglycans of secretory immunoglobulins A from human milk. Eur J Biochem. 1982 Jul;125(2):383–388. doi: 10.1111/j.1432-1033.1982.tb06694.x. [DOI] [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Ishii I., Ishihara H., Mori M., Tejima S., Jefferis R., Endo S., Arata Y. Comparative structural study of the N-linked oligosaccharides of human normal and pathological immunoglobulin G. Biochemistry. 1987 Feb 24;26(4):1137–1144. doi: 10.1021/bi00378a023. [DOI] [PubMed] [Google Scholar]
- Takasaki S., Mizuochi T., Kobata A. Hydrazinolysis of asparagine-linked sugar chains to produce free oligosaccharides. Methods Enzymol. 1982;83:263–268. doi: 10.1016/0076-6879(82)83019-x. [DOI] [PubMed] [Google Scholar]
- Townsend R. R., Hardy M. R., Lee Y. C. Separation of oligosaccharides using high-performance anion-exchange chromatography with pulsed amperometric detection. Methods Enzymol. 1989;179:65–76. doi: 10.1016/0076-6879(89)79114-x. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Yogeeswaran G., Salk P. L. Metastatic potential is positively correlated with cell surface sialylation of cultured murine tumor cell lines. Science. 1981 Jun 26;212(4502):1514–1516. doi: 10.1126/science.7233237. [DOI] [PubMed] [Google Scholar]